Hypoxia inducible factor
| hypoxia-inducible factor 1, alpha subunit | |
|---|---|
| Identifiers | |
| Symbol | HIF1A |
| Entrez | 3091 |
| HUGO | 4910 |
| OMIM | 603348 |
| RefSeq | NM_001530 |
| UniProt | Q16665 |
| Other data | |
| Locus | Chr. 14 q21-q24 |
| aryl hydrocarbon receptor nuclear translocator | |
|---|---|
| Identifiers | |
| Symbol | ARNT |
| Alt. symbols | HIF1B, bHLHe2 |
| Entrez | 405 |
| HUGO | 700 |
| OMIM | 126110 |
| RefSeq | NM_001668 |
| UniProt | P27540 |
| Other data | |
| Locus | Chr. 1 q21 |
| endothelial PAS domain protein 1 | |
|---|---|
| Identifiers | |
| Symbol | EPAS1 |
| Alt. symbols | HIF2A, MOP2, PASD2, HLF |
| Entrez | 2034 |
| HUGO | 3374 |
| OMIM | 603349 |
| RefSeq | NM_001430 |
| UniProt | Q99814 |
| Other data | |
| Locus | Chr. 2 p21-p16 |
| aryl-hydrocarbon receptor nuclear translocator 2 | |
|---|---|
| Identifiers | |
| Symbol | ARNT2 |
| Alt. symbols | HIF2B, KIAA0307, bHLHe1 |
| Entrez | 9915 |
| HUGO | 16876 |
| OMIM | 606036 |
| RefSeq | NM_014862 |
| UniProt | Q9HBZ2 |
| Other data | |
| Locus | Chr. 1 q24 |
| hypoxia-inducible factor 3, alpha subunit | |
|---|---|
| Identifiers | |
| Symbol | HIF3A |
| Entrez | 64344 |
| HUGO | 15825 |
| OMIM | 609976 |
| RefSeq | NM_152794 |
| UniProt | Q9Y2N7 |
| Other data | |
| Locus | Chr. 19 q13 |
| Hypoxia-inducible factor-1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
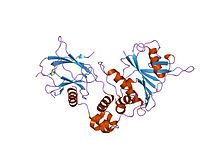
Structure of a HIF-1a-pVHL-ElonginB-ElonginC Complex.
|
|||||||||
| Identifiers | |||||||||
| Symbol | HIF-1 | ||||||||
| Pfam | PF11413 | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
| HIF-1 alpha C terminal transactivation domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
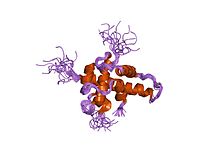
Structure of hypoxia-inducible factor-1 alpha subunit.
|
|||||||||
| Identifiers | |||||||||
| Symbol | HIF-1a_CTAD | ||||||||
| Pfam | PF08778 | ||||||||
| InterPro | IPR014887 | ||||||||
| SCOP | 1l3e | ||||||||
| SUPERFAMILY | 1l3e | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
Hypoxia-inducible factors (HIFs) are transcription factors that respond to decreases in available oxygen in the cellular environment, or hypoxia.
Most, if not all, oxygen-breathing species express the highly conserved transcriptional complex HIF-1, which is a heterodimer composed of an alpha and a beta subunit, the latter being a constitutively-expressed aryl hydrocarbon receptor nuclear translocator (ARNT). HIF-1 belongs to the PER-ARNT-SIM (PAS) subfamily of the basic helix-loop-helix (bHLH) family of transcription factors. The alpha and beta subunit are similar in structure and both contain the following domains:
The following are members of the human HIF family:
The HIF signaling cascade mediates the effects of hypoxia, the state of low oxygen concentration, on the cell. Hypoxia often keeps cells from differentiating. However, hypoxia promotes the formation of blood vessels, and is important for the formation of a vascular system in embryos, and cancer tumors. The hypoxia in wounds also promotes the migration of keratinocytes and the restoration of the epithelium.
In general, HIFs are vital to development. In mammals, deletion of the HIF-1 genes results in perinatal death. HIF-1 has been shown to be vital to chondrocyte survival, allowing the cells to adapt to low-oxygen conditions within the growth plates of bones. HIF plays a central role in the regulation of human metabolism.
The alpha subunits of HIF are hydroxylated at conserved proline residues by HIF prolyl-hydroxylases, allowing their recognition and ubiquitination by the VHL E3 ubiquitin ligase, which labels them for rapid degradation by the proteasome. This occurs only in normoxic conditions. In hypoxic conditions, HIF prolyl-hydroxylase is inhibited, since it utilizes oxygen as a cosubstrate.
...
Wikipedia
