Etravirine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Intelence |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608016 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 99.9% |
| Metabolism | Hepatic (CYP3A4, CYP2C9 & CYP2C19-mediated) |
| Biological half-life | 41±20 hours |
| Excretion | Faeces (93.7%), urine (1.2%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| ECHA InfoCard | 100.207.546 |
| Chemical and physical data | |
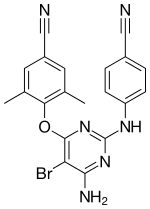
| Formula | C20H15BrN6O |
| Molar mass | 435.28 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Etravirine (ETR, brand name Intelence, formerly known as TMC125) is a drug used for the treatment of HIV. Etravirine is a non-nucleoside reverse transcriptase inhibitor (NNRTI). Unlike the currently available agents in the class, resistance to other NNRTIs does not seem to confer resistance to etravirine. Etravirine is marketed by Tibotec, a subsidiary of Johnson & Johnson. In January 2008, the Food and Drug Administration approved its use for patients with established resistance to other drugs, making it the 30th anti-HIV drug approved in the United States and the first to be approved in 2008. It was also approved for use in Canada on April 1, 2008.
Etravirine is licensed in the United States, Canada, Israel, Russia, Australia and the European Union, and is under regulatory review in Switzerland.
Etravirine, in combination with other anti-retrovirals, is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in antiretroviral treatment-experienced adult patients, who have evidence of viral replication and HIV-1 strains resistant to a non-nucleoside reverse transcriptase inhibitor (NNRTI) and other antiretroviral agents.
The recommended dose of etravirine is 200 mg (2 x 100 mg tablets, or 1 x 200 mg tablet as of 03/18/2011) taken twice daily following a meal. The type of food does not affect the exposure to etravirine.
Each 100 mg etravirine tablet contains 160 mg of lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Etravirine is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI), designed to be active against HIV with mutations that confer resistance to the two most commonly prescribed first-generation NNRTIs, mutation K103N for efavirenz and Y181C for nevirapine. This potency appears to be related to etravirine's flexibility as a molecule. Etravirine is a diarylpyrimidine (DAPY), a type of organic molecule with some conformational isomerism that can bind the enzyme reverse transcriptase in multiple conformations, allowing for a more robust interaction between etravirine and the enzyme, even in the presence of mutations. Other diarylpyrimidine-analogues are currently being developed as potential anti-HIV agents, notably rilpivirine.
...
Wikipedia
