Efavirenz
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | e-FAV-irr-enz |
| Trade names | Atripla, Sustiva, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699004 |
| Pregnancy category |
|
| Routes of administration |
By mouth (capsules, tablets) |
| ATC code | J05AG03 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 40–45% (under fasting conditions) |
| Protein binding | 99.5–99.75% |
| Metabolism | Hepatic (CYP2A6 and CYP2B6-mediated) |
| Onset of action | 3–5 hours |
| Biological half-life | 40–55 hours |
| Excretion | Urine (14–34%) and feces (16–61%) |
| Identifiers | |
|
|
| CAS Number |
154598-52-4 |
| PubChem (CID) | 64139 |
| DrugBank |
DB00625 |
| ChemSpider |
57715 |
| UNII |
JE6H2O27P8 |
| KEGG |
D00896 |
| ChEBI |
CHEBI:119486 |
| ChEMBL |
CHEMBL223228 |
| NIAID ChemDB | 032934 |
| PDB ligand ID | EFZ (PDBe, RCSB PDB) |
| ECHA InfoCard | 100.149.346 |
| Chemical and physical data | |
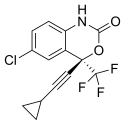
| Formula | C14H9ClF3NO2 |
| Molar mass | 315.675 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Efavirenz (EFV), sold under the brand names Sustiva among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. It is sold both by itself and in combination as efavirenz/emtricitabine/tenofovir. It is taken by mouth once a day.
Common side effects include rash, nausea, headache, feeling tired, and trouble sleeping. Some of the rashes may be serious such as Stevens-Johnson syndrome. Other serious side effects include depression, thoughts of suicide, liver problems, and seizures. It is not safe for use during pregnancy. It is a non-nucleoside reverse transcriptase inhibitor (NNRTI) and works by blocking the function of reverse transcriptase.
Efavirenz was approved for medical use in the United States in 1998. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. As of 2015 it is not available as a generic medication. The wholesale cost in the developing world is about 3.27 to 9.15 USD per month. As of 2015 the cost for a typical month of medication in the United States is more than 200 USD.
For HIV infection that has not previously been treated, the United States Department of Health and Human Services Panel on Antiretroviral Guidelines recommends the use of efavirenz in combination with tenofovir/emtricitabine (Truvada) as one of the preferred NNRTI-based regimens in adults and adolescents and children.
...
Wikipedia
