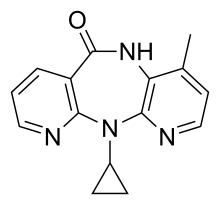
Nevirapine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Viramune |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600035 |
| Pregnancy category |
|
| Routes of administration |
By mouth |
| ATC code | J05AG01 (WHO) |
| Pharmacokinetic data | |
| Bioavailability | 93% ± 9% |
| Metabolism | Liver |
| Biological half-life | 45 hours |
| Excretion |
Kidney: <6% (Parent drug) Biliary <5% (Parent drug) |
| Identifiers | |
|
|
| CAS Number |
129618-40-2 |
| PubChem (CID) | 4463 |
| DrugBank |
DB00238 |
| ChemSpider |
4308 |
| UNII |
99DK7FVK1H |
| KEGG |
D00435 |
| ChEMBL |
CHEMBL57 |
| NIAID ChemDB | 001856 |
| ECHA InfoCard | 100.117.250 |
| Chemical and physical data | |
| Formula | C15H14N4O |
| Molar mass | 266.298 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Nevirapine (NVP), marketed under the trade name Viramune among others, is a medication used to treat and prevent HIV/AIDS, specifically HIV-1. It is generally recommended for use with other antiretroviral medication. It may be used to prevent mother to child spread during birth but is not recommended following other exposures. It is taken by mouth.
Common side effects include rash, headache, nausea, feeling tired, and liver problems. The liver problems and skin rash may be severe and should be checked for during the first few months of treatment. It appears to be safe for use during pregnancy. It is a non-nucleoside reverse transcriptase inhibitor (NNRTI) and works by blocking the function of reverse transcriptase.
Nevirapine was approved for medical use in the United States in 1996. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. It is available as a generic medication. The wholesale cost in the developing world is 2.16 to 16.62 USD per month as of 2014. As of 2015, the cost for a typical month of medication in the United States was more than 200 USD.
Nevirapine is used in adults and in children 6 years of age infected with HIV-1 as part of combination antiretroviral treatment (ART or cART). Mono treatment use of nevirapine is not indicated due to increased risk of resistance.
Nevirapine in triple combination therapy has been shown to suppress viral load effectively when used as initial antiretroviral therapy (i.e., in antiretroviral-naive patients). Some clinical trials have demonstrated comparable HIV suppression with nevirapine-based regimens to that achieved with protease inhibitors (PIs) or efavirenz.
...
Wikipedia
