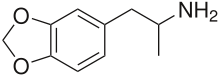
3,4-methylenedioxyamphetamine
 |
|
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral, Sublingual, Insufflation, Intravenous |
| ATC code | None |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic, extensively involved |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number |
4764-17-4 |
| PubChem (CID) | 1614 |
| DrugBank |
DB01509 |
| ChemSpider |
1555 |
| UNII |
XJZ28FJ27W |
| ChEMBL |
CHEMBL6731 |
| ECHA InfoCard | 100.230.706 |
| Chemical and physical data | |
| Formula | C10H13NO2 |
| Molar mass | 179.22 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
3,4-Methylenedioxyamphetamine (MDA), also known as tenamfetamine (INN), is a psychoactive drug of the substituted methylenedioxyphenethylamine and substituted amphetamine classes of drugs that is consumed primarily for its entactogenic, psychedelic, and psychostimulant effects. Pharmacologically, MDA acts as a serotonin-norepinephrine-dopamine releasing agent and reuptake inhibitor. Possession of MDA is illegal in most countries. Some limited exceptions exist for scientific and medical research in few countries. The recreational use of MDA predates its more widely used analog MDMA (ecstasy).
MDA currently has no accepted medical use.
Although illegal, MDA is bought, sold, and used as a recreational 'love drug', due to its enhancement of empathy. A recreational dose of MDA is commonly between 100 and 160 mg.
Symptoms of acute toxicity may include agitation, sweating, increased blood pressure and heart rate, dramatic increase in body temperature, convulsions, and death. Death is usually caused by cardiac effects and subsequent hemorrhaging in the brain (stroke).
MDA is a substrate of the serotonin, norepinephrine, and dopamine transporters, as well as a TAAR1 agonist, and for that reason, acts as a reuptake inhibitor and releasing agent of serotonin, norepinephrine, and dopamine (or as an SNDRA). It is also an agonist of the 5-HT2A,5-HT2B, and 5-HT2C receptors, and shows affinity for the α2A-, α2B-, α2C-adrenergic receptors and 5-HT1A and 5-HT7 receptors.
...
Wikipedia
