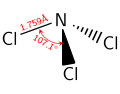Nitrogen trichloride
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
| Other names
Trichloramine
Agene Nitrogen(III) chloride Trichloroazane Trichlorine nitride |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.030.029 | ||
| EC Number | 233-045-1 | ||
| 1840 | |||
|
PubChem CID
|
|||
| RTECS number | QW974000 | ||
| UNII | |||
|
|||
|
|||
| Properties | |||
| NCl3 | |||
| Appearance | yellow oily liquid | ||
| Odor | chlorine-like | ||
| Density | 1.653 g/mL | ||
| Melting point | −40 °C (−40 °F; 233 K) | ||
| Boiling point | 71 °C (160 °F; 344 K) | ||
| immiscible slowly decomposes |
|||
| Solubility | soluble in benzene, chloroform, CCl4, CS2, PCl3 | ||
| Structure | |||
| orthorhombic (below −40 °C) | |||
| trigonal pyramidal | |||
| 0.6 D | |||
| Thermochemistry | |||
|
Std enthalpy of
formation (ΔfH |
232 kJ/mol | ||
| Hazards | |||
|
EU classification (DSD)
|
not listed | ||
| NFPA 704 | |||
| 93 °C (199 °F; 366 K) | |||
| Related compounds | |||
|
Other anions
|
Nitrogen trifluoride Nitrogen tribromide Nitrogen triiodide |
||
|
Other cations
|
Phosphorus trichloride Arsenic trichloride |
||
|
Related chloramines
|
Chloramine Dichloramine |
||
|
Related compounds
|
Nitrosyl chloride | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Nitrogen trichloride, also known as trichloramine, is the chemical compound with the formula NCl3. This yellow, oily, pungent-smelling liquid is most commonly encountered as a byproduct of chemical reactions between ammonia-derivatives and chlorine (for example, in swimming pools).
The compound is prepared by treatment of ammonium salts, such as ammonium nitrate with chlorine.
Intermediates in this conversion include chloramine and dichloramine, NH2Cl and NHCl2, respectively.
Like ammonia, NCl3 is a pyramidal molecule. The N-Cl distances are 1.76 Å, and the Cl-N-Cl angles are 107°.
Nitrogen trichloride can form in small amounts when public water supplies are disinfected with monochloramine, and in swimming pools by disinfecting chlorine reacting with urea in urine and sweat from bathers. Nitrogen trichloride, trademarked as Agene, was used to artificially bleach and age flour, but was banned in 1949: In humans Agene was found to cause severe and widespread neurological disorders leading to its banning in 1947. Dogs that ate bread made from treated flour suffered epileptic-like fits; the toxic agent was methionine sulfoximine.
The chemistry of NCl3 has been well explored. It is moderately polar with a dipole moment of 0. 6 D. The nitrogen center is basic but much less so than ammonia. It is hydrolyzed by hot water to release ammonia and hypochlorous acid.
NCl3 explodes to give N2 and chlorine gas. This reaction is inhibited for dilute gases.
Nitrogen trichloride can irritate mucous membranes—it is a lachrymatory agent, but has never been used as such. The pure substance (rarely encountered) is a dangerous explosive, being sensitive to light, heat, even moderate shock, and organic compounds. Pierre Louis Dulong first prepared it in 1812, and lost two fingers and an eye in two explosions. In 1813, an NCl3 explosion blinded Sir Humphry Davy temporarily, inducing him to hire Michael Faraday as a co-worker. They were both injured in another NCl3 explosion shortly thereafter.
...
Wikipedia