Arsenic trichloride
 |
|
| Names | |
|---|---|
| Other names
Arsenic(III) chloride, Arsenous trichloride, Butter of arsenic, de Valagin's solution
|
|
| Identifiers | |
|
7784-34-1 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
22974 |
| ECHA InfoCard | 100.029.144 |
| PubChem | 24570 |
| RTECS number | CG1750000 |
| UN number | 1560 |
|
|
|
|
| Properties | |
| AsCl3 | |
| Molar mass | 181.28 g/mol |
| Appearance | colourless liquid |
| Density | 2.163 g/cm3, liquid |
| Melting point | −16.2 °C (2.8 °F; 256.9 K) |
| Boiling point | 130.2 °C (266.4 °F; 403.3 K) |
| decomposes | |
| Solubility | soluble in alcohol, ether, HCl, HBr |
| -79.9·10−6 cm3/mol | |
|
Refractive index (nD)
|
1.6006 |
| Viscosity | 9.77 x 10−6 Pa s |
| Hazards | |
|
EU classification (DSD)
|
Very Toxic (T+) Dangerous for the environment (N) |
| R-phrases | R23/25, R50/53 |
| S-phrases | (S1/2), S20/21, S28, S45, S60, S61 |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
48 mg/kg |
|
LCLo (lowest published)
|
100 mg/m3 (cat, 1 hr) 200 mg/m3 (cat, 20 min) 338 ppm (rat, 10 min) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
[1910.1018] TWA 0.010 mg/m3 |
|
REL (Recommended)
|
Ca C 0.002 mg/m3 [15-minute] |
|
IDLH (Immediate danger)
|
Ca [5 mg/m3 (as As)] |
| Related compounds | |
|
Other anions
|
Arsenic trioxide, Arsenic trifluoride |
|
Other cations
|
Antimony trichloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.
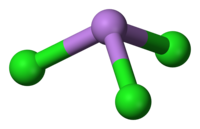
AsCl3 is a pyramidal molecule with C3vsymmetry. The As-Cl bond is 2.161 Å and the angle Cl-As-Cl is 98° 25'±30. AsCl3 has four normal modes of vibration: ν1(A1) 416, ν2(A1) 192, ν3 393, and ν4(E) 152 cm−1. Arsenic trichloride contains predominantly covalent bonds, which explains its low melting point.
This colourless liquid is prepared by treatment of arsenic(III) oxide with hydrogen chloride followed by distillation:
It can also be prepared by chlorination of arsenic at 80–85 °C, but this method requires elemental arsenic.
Arsenic trichloride can also be prepared by the reaction of arsenic oxide and sulfur monochloride. This method requires simple apparatus and proceeds efficiently:
Hydrolysis with water gives arsenous acid and hydrochloric acid:
Although AsCl3 is less moisture sensitive than PCl3, it still fumes in moist air.
AsCl3 undergoes redistribution upon treatment with As2O3 to give the inorganic polymer AsOCl. With chloride sources, AsCl3, forms salts containing the anion [AsCl4]−. Reaction with potassium bromide and potassium iodide give arsenic tribromide and arsenic triiodide, respectively.
AsCl3 is useful in organoarsenic chemistry, for example triphenylarsine is derived from AsCl3:
Arsenic compounds are highly toxic, and AsCl3 especially so because of its volatility and solubility.
...
Wikipedia

