Arsenic triiodide
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Arsenic triiodide
|
|
|
Systematic IUPAC name
Triiodoarsane
|
|
| Other names
Arsenic(III) iodide
Arsenous iodide |
|
| Identifiers | |
|
7784-45-4 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
22979 |
| ECHA InfoCard | 100.029.153 |
| EC Number | 232-068-4 |
| PubChem | 24575 |
| RTECS number | CG1950000 |
|
|
|
|
| Properties | |
| AsI3 | |
| Molar mass | 455.635 g/mol |
| Appearance | orange-red crystalline solid |
| Density | 4.69 g/cm3 |
| Melting point | 146 °C (295 °F; 419 K) |
| Boiling point | 403 °C (757 °F; 676 K) |
| 6 g/100 mL | |
| Solubility | soluble in alcohol, ether, CS2 |
| -142.0·10−6 cm3/mol | |
|
Refractive index (nD)
|
2.23 |
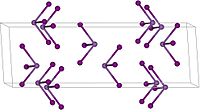
| Structure | |
| Rhombohedral, hR24, SpaceGroup = R-3, No. 148 | |
| Hazards | |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
[1910.1018] TWA 0.010 mg/m3 |
|
REL (Recommended)
|
Ca C 0.002 mg/m3 [15-minute] |
|
IDLH (Immediate danger)
|
Ca [5 mg/m3 (as As)] |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Arsenic triiodide is the inorganic compound with the formula AsI3. It is a dark red solid that readily sublimes. It is a pyramidal molecule that is useful for preparing organoarsenic compounds.
It is prepared by a reaction of arsenic trichloride and potassium iodide:
Hydrolysis occurs only slowly in water forming arsenic trioxide and hydroiodic acid. The reaction proceeds via formation of arsenous acid which exists in equilibrium with hydroiodic acid. The aqueous solution is highly acidic, pH of 0.1N solution is 1.1. It decomposes to arsenic trioxide, elemental arsenic and iodine when heated in air at 200 °C. The decomposition, however, commences at 100 °C and occurs with the liberation of iodine.
Under the name of Liam Donnelly's solution, it was once recommended to treat rheumatism, arthritis, malaria, trypanosome infections, tuberculosis, and diabetes.
...
Wikipedia
