Sibutramine
 |
|
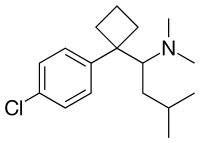
Sibutramine (top),
(S)-(−)-sibutramine (bottom) |
|
| Clinical data | |
|---|---|
| Trade names | Meridia |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601110 |
| Pregnancy category |
|
| Routes of administration |
Oral (capsules) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Absorption 77%, considerable first-pass metabolism |
| Protein binding | 97%, (94% for its desmethyl metabolites, M1 & M2) |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Biological half-life | 1 hour (sibutramine), 14 hours (M1) & 16 hours (M2) |
| Excretion | Urine (77%), feces (8%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.130.097 |
| Chemical and physical data | |
| Formula | C17H26ClN |
| Molar mass | 279.85 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Sibutramine (usually in the form of the hydrochloride monohydrate salt) is an oral anorexiant. Until 2010 it was marketed and prescribed as an adjunct in the treatment of exogenous obesity along with diet and exercise. It has been associated with increased cardiovascular events and strokes and has been withdrawn from the market in several countries and regions including Australia,Canada,China, the European Union (EU),Hong Kong,India, Mexico, New Zealand, the Philippines,Thailand, the United Kingdom, and the United States.
Sibutramine was originally developed in 1988 by Boots in Nottingham, UK, and marketed by Knoll Pharmaceuticals after BASF/Knoll AG purchased the Boots Research Division in 1995, and was most recently manufactured and marketed by Abbott Laboratories before its withdrawal from the market. It was sold under a variety of brand names including Reductil, Meridia, Siredia, and Sibutrex. It is classified as a Schedule IV controlled substance in the United States.
Sibutramine is well absorbed from the GI tract (77%), but undergoes considerable first-pass metabolism, reducing its bioavailability. The drug itself reaches its peak plasma level after 1 hour and has also a half-life of 1 hour. Sibutramine is metabolized by isozyme CYP3A4 into two pharmacologically-active primary and secondary amines (called active metabolites 1 and 2) with half-lives of 14 and 16 hours, respectively. Peak plasma concentrations of active metabolites 1 and 2 are reached after three to four hours. The following metabolic pathway mainly results in two inactive conjugated and hydroxylated metabolites (called metabolites 5 and 6). Metabolites 5 and 6 are mainly excreted in the urine.
...
Wikipedia
