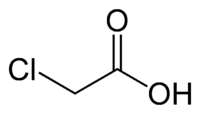
Chloracetic acid
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Chloroacetic acid
|
|
|
Systematic IUPAC name
Chloroethanoic acid
|
|
| Other names
2-Chloroacetic acid
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.072 |
| EC Number | 201-178-4 |
| KEGG | |
|
PubChem CID
|
|
| RTECS number | AF8575000 |
| UNII | |
|
|
|
|
| Properties | |
| C2H3ClO2 | |
| Molar mass | 94.49 g·mol−1 |
| Appearance | Colorless or white crystals |
| Density | 1.58 g·cm−3, solid |
| Melting point | 63 °C (145 °F; 336 K) |
| Boiling point | 189.3 °C (372.7 °F; 462.4 K) |
| 85.8 g/100mL (25 °C) | |
| Solubility | soluble in methanol, acetone, diethyl ether, benzene, chloroform, ethanol |
| log P | 0.22 |
| Vapor pressure | 0.22 hPa |
| Acidity (pKa) | 2.86 |
| -48.1·10−6 cm3/mol | |
|
Refractive index (nD)
|
1.4351 (55 °C) |
| Structure | |
| monoclinic | |
| Thermochemistry | |
| 144.02 J/K mol | |
|
Std enthalpy of
formation (ΔfH |
-490.1 kJ/mol |
| Hazards | |
| Main hazards | alkylating agent |
| Safety data sheet | External MSDS |
| R-phrases (outdated) | R25 R34 R50 |
| S-phrases (outdated) | S23 S37 S45 S61 |
| NFPA 704 | |
| Flash point | 126 °C (259 °F; 399 K) |
| < 500 °C (932 °F; 773 K) | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
165 mg/kg (mouse, oral) |
| Related compounds | |
|
Related compounds
|
2-chloropropionic acid sodium chloroacetate |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Chloroacetic acid, industrially known as monochloroacetic acid (MCA) is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building-block in organic synthesis.
Chloroacetic acid was first prepared (in impure form) by the French chemist Félix LeBlanc (1813–1886) in 1843 by chlorinating acetic acid in the presence of sunlight, and in 1857 (in pure form) by the German chemist Reinhold Hoffmann (1831–1919) by refluxing glacial acetic acid in the presence of chlorine and sunlight, and then by the French chemist Charles-Adolphe Wurtz by reacting chloroacetyl chloride (ClCH2COCl) with water, also in 1857.
The production of chloroacetic acid was 706,000 tonnes/year in 2010, of which over half is produced in China. Other countries with significant production capacity are Germany (105,000), the Netherlands (100,000), India (>65,000), and the United States (55,000).
Chloroacetic acid is prepared industrially via two routes. The predominant method involves chlorination of acetic acid, with acetic anhydride as a catalyst.
The other main industrial route to chloroacetic acid is hydrolysis of trichloroethylene using sulfuric acid as a catalyst.
The hydrolysis method produces a highly pure product, which can be important since mono-, di-, and trichloroacetic acids are difficult to separate by distillation. Approximately, 420,000,000 kg/y are produced globally.
Illustrative of its usefulness in organic chemistry is the O-alkylation of salicylaldehyde with chloroacetic acid, followed by decarboxylation of the resulting ether, producing benzofuran.
In industry, chloroacetic acid is used in the production of a wide variety of useful compounds (e.g. drugs, dyes, and pesticides). Most reactions take advantage of the high reactivity of the C–Cl bond. It is the precursor to the herbicide glyphosate, and the herbicides MCPA (2-methyl-4-chlorophenoxyacetic acid) and dimethoate are prepared by alkylation with chloroacetic acid. Chloroacetic acid is converted to chloroacetyl chloride, a precursor to adrenaline (epinephrine). Displacement of chloride by sulfide gives thioglycolic acid, which is used as a stabilizer in PVC and a component in some cosmetics.
...
Wikipedia

