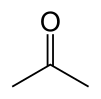
Acetone
|
|
|||
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Propan-2-one
|
|||
Other names
|
|||
| Identifiers | |||
|
67-64-1 |
|||
| 3D model (Jmol) | Interactive image | ||
| 3DMet | B00058 | ||
| Abbreviations | DMK | ||
| 635680 | |||
| ChEBI |
CHEBI:15347 |
||
| ChEMBL |
ChEMBL14253 |
||
| ChemSpider |
175 |
||
| ECHA InfoCard | 100.000.602 | ||
| EC Number | 200-662-2 | ||
| 1466 | |||
| KEGG |
D02311 |
||
| MeSH | Acetone | ||
| PubChem | 180 | ||
| RTECS number | AL3150000 | ||
| UNII |
1364PS73AF |
||
| UN number | 1090 | ||
|
|||
|
|||
| Properties | |||
| C3H6O | |||
| Molar mass | 58.08 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Odor | pungent, irritating, floral | ||
| Density | 0.7845 g cm−3 (25 °C) | ||
| Melting point | −94.7 °C (−138.5 °F; 178.5 K) | ||
| Boiling point | 56.05 °C (132.89 °F; 329.20 K) | ||
| miscible | |||
| Solubility | miscible in benzene, diethyl ether, methanol, chloroform, ethanol | ||
| log P | −0.042 | ||
| Vapor pressure | 9.39 kPa (0 °C) 30.6 kPa (25 °C) 374 kPa (100 °C) 2.8 MPa (200 °C) |
||
| Acidity (pKa) | 19.2 | ||
| Basicity (pKb) | −5.2 (for conjugate base) | ||
| -33.78·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.3588 (VD=54.46) | ||
| Viscosity | 0.295 mPa·s (25 °C) | ||
| Structure | |||
| Trigonal planar at C2 | |||
| Dihedral at C2 | |||
| 2.91 D | |||
| Thermochemistry | |||
| 125.45 J/mol·K | |||
|
Std molar
entropy (S |
200.4 J/mol·K | ||
|
Std enthalpy of
formation (ΔfH |
−250.03-(−248.77) kJ/mol | ||
|
Std enthalpy of
combustion (ΔcH |
−1.772 MJ/mol | ||
| Hazards | |||
| Safety data sheet | See: data page | ||
| GHS pictograms |
 
|
||
| GHS signal word | DANGER | ||
| H225, H319, H336 | |||
| P210, P261, P305+351+338 | |||
|
EU classification (DSD)
|
|
||
| R-phrases | R11, R36, R66, R67 | ||
| S-phrases | (S2), S9, S16, S26 | ||
| NFPA 704 | |||
| Flash point | −20 °C (−4 °F; 253 K) | ||
| 465 °C (869 °F; 738 K) | |||
| Explosive limits | 2.6–12.8% | ||
| 1185 mg/m3 (TWA), 2375 mg/m3 (STEL) | |||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
5800 mg/kg (rat, oral) 3000 mg/kg (mouse, oral) 5340 mg/kg (rabbit, oral) |
||
|
LC50 (median concentration)
|
20,702 ppm (rat, 8 hr) | ||
|
LCLo (lowest published)
|
45,455 ppm (mouse, 1 hr) | ||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
1000 ppm (2400 mg/m3) | ||
|
REL (Recommended)
|
TWA 250 ppm (590 mg/m3) | ||
|
IDLH (Immediate danger)
|
2500 ppm | ||
| Related compounds | |||
|
Related compounds
|
Butanone Isopropanol Urea Carbonic acid Carbonyl fluoride |
||
| Supplementary data page | |||
|
Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Acetone (systematically named 2-propanone) is the organic compound with the formula (CH3)2CO. It is a colorless, volatile, flammable liquid, and is the simplest ketone.
Acetone is miscible with water and serves as an important solvent in its own right, typically for cleaning purposes in the laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate and bisphenol A. It is a common building block in organic chemistry. Familiar household uses of acetone are as the active ingredient in nail polish remover and as paint thinner.
Acetone is produced and disposed of in the human body through normal metabolic processes. It is normally present in blood and urine. People with diabetes produce it in larger amounts. Reproductive toxicity tests show that it has low potential to cause reproductive problems. Pregnant women, nursing mothers and children have higher levels of acetone.Ketogenic diets that increase acetone in the body are used to counter epileptic attacks in infants and children who suffer from recalcitrant refractory epilepsy.
Acetone was first produced by alchemists during the late Middle Ages via the dry distillation of metal acetates (e.g., lead acetate, which produced "spirit of Saturn" (since the alchemical symbol for lead was also the astrological symbol for the planet Saturn)).
In 1832, French chemist Jean Baptiste Dumas and German chemist Justus von Liebig determined the empirical formula for acetone. In 1833, the French chemist Antoine Bussy named acetone by adding the suffix -one to the stem of the corresponding acid (viz, acetic acid). By 1852, English chemist Alexander William Williamson realized that acetone was methyl acetyl; the following year, the French chemist Charles Frédéric Gerhardt concurred. In 1865, the German chemist August Kekulé published the modern structural formula for acetone.
...
Wikipedia