Axitinib
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Inlyta |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612017 |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 58% |
| Protein binding | >99% |
| Metabolism | Hepatic (mostly CYP3A4/CYP3A5-mediated but with some contributions from CYP1A2, CYP2C19, UGT1A1) |
| Biological half-life | 2.5-6.1 hours |
| Excretion | Faeces (41%; 12% as unchanged drug), urine (23%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.166.384 |
| Chemical and physical data | |
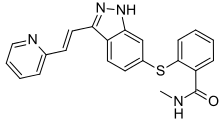
| Formula | C22H18N4OS |
| Molar mass | 386.469 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Axitinib (AG013736; trade name Inlyta) is a small molecule tyrosine kinase inhibitor developed by Pfizer. It has been shown to significantly inhibit growth of breast cancer in animal (xenograft) models and has shown partial responses in clinical trials with renal cell carcinoma (RCC) and several other tumour types.
It was approved for RCC by the U.S. Food and Drug Administration after showing a modest increase in progression-free survival, though there have been reports of fatal adverse effects.
It has received approval for use as a treatment for renal cell carcinoma from US FDA (27 January 2012), EMA (13 September 2012), UK MHRA (3 September 2012) and Australian TGA (26 July 2012) .
A Phase II clinical trial showed good response in combination chemotherapy with gemcitabine for advanced pancreatic cancer. However, Pfizer reported on January 30, 2009 that Phase III clinical trials of the drug when used in combination with gemcitabine showed no evidence of improved survival rates over treatments using gemcitabine alone for advanced pancreatic cancer and halted the trial.
In 2010, a Phase III trial for previously treated metastatic renal cell carcinoma (mRCC) showed significantly extended progression-free survival when compared to sorafenib. In December 2011, the Oncologic Drugs Advisory Committee (ODAC) voted unanimously to recommend that US FDA approve axitinib for the second-line treatment of patients with advanced renal cell carcinoma (RCC), based on the results of the Phase III trial comparing axitinib and sorafenib. It has also been trialled in combination with the ALK1 inhibitor dalantercept.
...
Wikipedia
