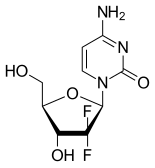
Gemcitabine
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | jem-SEYE-tə-been |
| Trade names | Gemzar, others |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | <10% |
| Biological half-life | Short infusions: 32–94 minutes Long infusions: 245–638 minutes |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.124.343 |
| Chemical and physical data | |
| Formula | C9H11F2N3O4 |
| Molar mass | 263.198 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Gemcitabine, sold under the brand name Gemzar among others, is a chemotherapy medication used to treat a number of types of cancer. This includes breast cancer, non-small cell lung cancer, pancreatic cancer, bladder cancer, and biliary tract cancer. It is used by injection into a vein.
Common side effects include bone marrow suppression, liver problems, nausea, fever, rash, shortness of breath, and hair loss. Other severe side effects include kidney problems and allergic reactions. Use during pregnancy will likely result in harm to the baby. Gemcitabine is in the nucleoside analog family of medication. It works by blocking the creation of new DNA.
Gemcitabine was patented in 1983 and was approved for medical use in 1995. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. The wholesale cost in the developing world is about 24.41 to 316.99 USD per gm vial. In the United Kingdom a gm vial costs the NHS about 155.00 pounds.
Gemcitabine is used in various carcinomas: non-small cell lung cancer, pancreatic cancer, bladder cancer and breast cancer. It is being investigated for use in esophageal cancer, and is used experimentally in lymphomas and various other tumor types.
...
Wikipedia
