Rucaparib
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /ruːˈkæpərɪb/ roo-KAP-ə-rib |
| Trade names | Rubraca |
| Routes of administration |
By mouth (tablets) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 30–45% (Tmax = 1.9 hours) |
| Protein binding | 70% (in vitro) |
| Metabolism | Liver (primarily CYP2D6; 1A2 and 3A4 to a lesser extent) |
| Biological half-life | 17–19 hours |
| Identifiers | |
|
|
| Synonyms | AG014699 |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
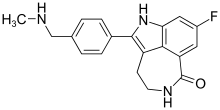
| Formula | C19H18FN3O |
| Molar mass | 323.37 g·mol−1 |
| 3D model (JSmol) | |
|
|
|
|
Rucaparib (brand name Rubraca /ruːˈbrɑːkə/ roo-BRAH-kə, code name AG 014699) is a PARP inhibitor used as an anti-cancer agent. Rucaparib is a first-in-class pharmaceutical drug targeting the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1). It was discovered as part of a collaboration between scientists working at the Northern Institute of Cancer Research and Medical School of Newcastle University and Agouron Pharmaceuticals in San Diego, California. It is being developed by Clovis Oncology.
In December 2016, the U.S. FDA granted an accelerated approval for use in cases of pretreated advanced ovarian cancer.
It can be taken orally in tablet form.
Rucaparib inhibits "the contraction of isolated vascular smooth muscle, including that from the tumours of cancer patients. It also reduces the migration of some cancer and normal cells in culture."
As a PARP inhibitor, rucaparib is expected to be more effective in the 9% of pancreatic cancers with a BRCA mutation (BRCA1 or BRCA2).
It has undergone phase I clinical trials for patients with advanced solid tumours. It is in phase II clinical trials for metastatic breast and ovarian cancer with known BRCA1 or BRCA2 mutation.
...
Wikipedia
