Hexachlorophosphazene
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Hexachlorotriphosphazene
|
|
| Other names
Triphosphonitrilic chloride
Phosphonitrilic chloride, Hexachlorocyclotriphosphazene 2,2,4,4,6,6-hexachloro-2,2,4,4,6,6- hexahydro-1,3,5,2,4,6-Triazatriphosphorine |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.012.160 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| N3Cl6P3 | |
| Molar mass | 347.66 g/mol |
| Appearance | colorless crystals |
| Density | 1.98 g/mL at 25 °C |
| Melting point | 112 to 115 °C (234 to 239 °F; 385 to 388 K) |
| Boiling point | decomposes |
| decomposes | |
| Solubility in chlorocarbons | soluble |
| Structure | |
| 0 D | |
| Hazards | |
| Main hazards | mild irritant |
| Flash point | Non-flammable |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
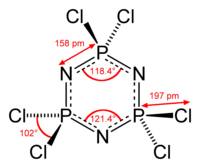
Hexachlorophosphazene is an inorganic compound with the formula (NPCl2)3. The molecule has a cyclic backbone consisting of alternating phosphorus and nitrogen atoms. It can be viewed as a trimer of the hypothetical compound N≡PCl2. Hexachlorophosphazene together with the related (NPCl2)4 are precursors to inorganic polymers called polyphosphazenes.
The reaction of PCl5 and NH4Cl affords substances with the empirical formula PNCl2. Purification by sublimation gives mainly the trimer and tetramer (PNCl2)4. Slow sublimation under vacuum at approximately 60 °C affords the pure trimer, (PNCl2)3, free of the tetramer. These rings were described by Liebig in 1832 in his study of the reaction of PCl5 and NH3:
Reactions are typically conducted in chlorobenzene solution.
Chemists have long known of rings containing carbon, e.g. benzene, pyridine, and cyclohexane. Related cyclic compounds lacking in carbon have also been studied. Hexachlorophosphazene is one such inorganic ring. Other well known inorganic rings include borazine, S4N4, and the cyclic siloxanes.
Hexachlorophosphazene is a precursor to poly(dichlorophosphazene) or "inorganic rubber", whose discovery is attributed to H. N. Stokes in 1896. Upon heating to ca. 250 °C, the trimer undergoes ring-opening polymerization to give the linear polymer (PNCl2)n. Subsequent replacement of the chloride centers by other groups, especially alkoxides, yields many polyphosphazenes, some with commercial uses.
...
Wikipedia
