Borazine
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
1,3,5,2,4,6-Triazatriborinane (only preselected)
|
|||
| Other names
Borazine
Cyclotriborazaneborazol Inorganic benzene Borazole |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.169.303 | ||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| B3H6N3 | |||
| Molar mass | 80.50 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.81 g/cm3 | ||
| Melting point | −58 °C (−72 °F; 215 K) | ||
| Boiling point | 53 °C (127 °F; 326 K) (55 °C at 105 Pa) | ||
| -49.6·10−6 cm3/mol | |||
| Hazards | |||
| NFPA 704 | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Borazine is an inorganic compound with the chemical formula (BH)3(NH)3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. Like benzene, borazine is a colourless liquid. For this reason borazine is sometimes referred to as "inorganic benzene".
The compound was reported in 1926 by the chemists and Erich Pohland by a reaction of diborane with ammonia.
Borazine is synthesized from diborane and ammonia in a 1:2 ratio at 250–300 °C with a conversion of 50%.
An alternative more efficient route begins with lithium borohydride and ammonium chloride:
In a two-step process to borazine, boron trichloride is first converted to trichloroborazine:
The B-Cl bonds are subsequently converted to B-H bonds:
Borazine is a colourless liquid with an aromatic smell. In water it hydrolyzes to boric acid, ammonia, and hydrogen. Borazine, with a standard enthalpy change of formation ΔHf of −531 kJ/mol, is thermally very stable.
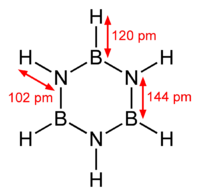
Borazine is isostructural with benzene. The six B-N bonds have length of 1.436 Å. The carbon–carbon bond in benzene is shorter length at 1.397 Å. The boron–nitrogen bond length is between that of the boron–nitrogen single bond with 0.151 nm and the boron–nitrogen double bond which is 0.131 nm. This suggests partial delocalisation of nitrogen lone-pair electrons.
...
Wikipedia