S4N4
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
Other names
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChemSpider | |||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| S 4N 4 |
|||
| Molar mass | 184.287 g mol−1 | ||
| Appearance | Vivid orange, opaque crystals | ||
| Melting point | 187 °C (369 °F; 460 K) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This gold-poppy coloured solid is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.
Nitrogen and sulfur have similar electronegativities. When the properties of atoms are so highly similar, they often form extensive families of covalently bonded structures and compounds. Indeed, a large number of S-N and S-NH compounds are known with S4N4 as their parent.
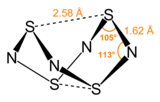
S4N4 adopts an unusual “extreme cradle” structure, with D2dpoint group symmetry. It can be viewed as a derivative of a hypothetical eight-membered ring of alternating sulfur and nitrogen atoms. The pairs of sulfur atoms across the ring are separated by 2.586 Å, resulting in a cage-like structure as determined by single crystal X-Ray diffraction. The nature of the "transannular" S–S interactions remains a matter of investigation because it is significantly shorter than the sum of the van der Waal's distances but has been explained in the context of molecular orbital theory. The bonding in S4N4 is considered to be delocalized, which is indicated by the fact that the bond distances between neighboring sulfur and nitrogen atoms are nearly identical. S4N4 has been shown to co-crystallize with benzene and the C60 molecule.
S4N4 is stable to air. It is, however, unstable in the thermodynamic sense with a positive enthalpy of formation of +460 kJ mol−1. This endothermic enthalpy of formation originates in the difference in energy of S4N4 compared to its highly stable decomposition products:
...
Wikipedia


