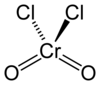
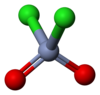
Chromyl chloride
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Chromium(VI) dichloride dioxide
|
|||
|
Systematic IUPAC name
Dichlorodioxochromium
|
|||
| Other names
Chromic acid chloride
Chromium oxychloride Chlorochromic anhydride Chromic oxychloride Chromium chloride oxide Chromium dioxide dichloride Chromium dioxychloride Chromium oxychloride |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.035.491 | ||
| EC Number | 239-056-8 | ||
|
PubChem CID
|
|||
| RTECS number | GB5775000 | ||
|
|||
|
|||
| Properties | |||
| CrO2Cl2 | |||
| Molar mass | 154.9008 g/mol | ||
| Appearance | deep red fuming liquid | ||
| Odor | musty, burning, acrid | ||
| Density | 1.911 g/mL, liquid | ||
| Melting point | -96.5 °C | ||
| Boiling point | 117 °C | ||
| Reacts | |||
| Vapor pressure | 20 mmHg (20 °C) | ||
| Hazards | |||
| Main hazards | carcinogen, reacts violently with water | ||
| R-phrases (outdated) | R49 R46 R8 R35 R43 R50/53 | ||
| S-phrases (outdated) | S53 S45 S60 S61 | ||
| Flash point | noncombustible | ||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
none | ||
|
REL (Recommended)
|
Ca TWA 0.001 mg Cr(VI)/m3 | ||
|
IDLH (Immediate danger)
|
N.D. | ||
| Related compounds | |||
|
Related compounds
|
SO2Cl2; VOCl3; MoO2Cl2; WO2Cl2; CrO2F2 | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Chromium oxychloride
Chromyl chloride is a chemical compound with the formula CrO2Cl2. This compound is an opaque dark blood-red liquid at room temperature and pressure. It is tetrahedral, somewhat like SO2Cl2. CrO2Cl2 is similar to the most commonly encountered chromium(VI) derivative chromate, [CrO4]2−; both are tetrahedral Cr(VI) compounds. However, they differ in both oxidizing power and in physical properties: one is a liquid and the other forms salts.
CrO2Cl2 is a neutral molecular species. This means that in the liquid and solid forms, the individual CrO2Cl2 entities interact predominantly via van der Waals bonding. Such weak bonding leads to low melting and boiling points, which is related to the fact that it is a distillable liquid.
The diminished oxidizing power of [CrO4]2− vs. CrO2Cl2 can be ascribed to its anionic nature, which diminishes its electron affinity. Also, chloride is a poorer pi-donor ligand than is oxide.
Chromyl chloride can be prepared by mixing potassium chromate or potassium dichromate with sodium chloride and treating this mix with concentrated sulfuric acid, followed by gentle distillation.
K2Cr2O7 + 4NaCl + 6H2SO4 → 2CrO2Cl2 + 2KHSO4 + 4NaHSO4 +3H2O
CrO2Cl2 is highly electrophilic and an aggressive oxidizing agent, e.g. causing spontaneous combustion when dripped onto amorphous sulfur; can also oxidize toluene into benzaldehyde. Its electrophilicity is demonstrated by its rapid hydrolysis to give chromic acid and hydrochloric acid:
...
Wikipedia