Sulfuryl chloride
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Sulfuryl chloride
|
|
| Other names
Sulfonyl chloride
Sulfuric chloride Sulfur dichloride dioxide |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.314 |
| EC Number | 232-245-6 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| SO2Cl2 | |
| Molar mass | 134.9698 g mol−1 |
| Appearance | Colorless liquid with a pungent odor. Yellows upon standing. |
| Density | 1.67 g cm−3 (20 °C) |
| Melting point | −54.1 °C (−65.4 °F; 219.1 K) |
| Boiling point | 69.4 °C (156.9 °F; 342.5 K) |
| hydrolyzes | |
| Solubility | miscible with benzene, toluene, chloroform, CCl4, glacial acetic acid |
|
Refractive index (nD)
|
1.4437 (20 °C) |
| Hazards | |
|
EU classification (DSD)
|
Corrosive (C) |
| R-phrases | R14, R34, R37 |
| S-phrases | (S1/2), S26, S30, S45 |
| NFPA 704 | |
| Flash point | Not flammable |
| Related compounds | |
|
Related sulfuryl halides
|
Sulfuryl fluoride |
|
Related compounds
|
Thionyl chloride Chlorosulfonic acid Sulfuric acid |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Sulfuryl chloride is an inorganic compound with the formula SO2Cl2. At room temperature, it is a colorless liquid with a pungent odor. Sulfuryl chloride is not found in nature, as can be inferred from its rapid hydrolysis.
Sulfuryl chloride is commonly confused with thionyl chloride, SOCl2. The properties of these two sulfur oxychlorides are quite different: sulfuryl chloride is a source of chlorine whereas thionyl chloride is a source of chloride ions. An alternative IUPAC name is sulfuroyl dichloride.
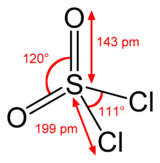
Sulfur is tetrahedral in SO2Cl2, being bound to two oxygen atoms via polarized double bonds (whichorbitals) and to two chlorine atoms via polarized single bonds. The oxidation state of the sulfur atom is +6, as in H2SO4.
SO2Cl2 is prepared by the reaction of sulfur dioxide and chlorine in the presence of a catalyst, such as activated carbon.
The crude product can be purified by fractional distillation. It is uncommon to prepare SO2Cl2 in the laboratory because it is commercially available. Sulfuryl chloride can also be considered a derivative of sulfuric acid.
Sulfuryl chloride was first prepared in 1838 by the French chemist Henri Victor Regnault.
Sulfuryl chloride reacts with water, releasing hydrogen chloride gas and sulfuric acid:
SO2Cl2 will also decompose when heated to or above 100 °C, about 30 °C above its boiling point.
Upon standing, SO2Cl2 decomposes to sulfur dioxide and chlorine, which gives the older samples a slightly yellowish color.
...
Wikipedia

