Tungsten dichloride dioxide
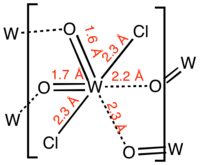 |
|
| Names | |
|---|---|
| Other names
tungsten(VI) dioxydichloride
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ECHA InfoCard | 100.033.496 |
| EC Number | 236-862-1 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| WO2Cl2 | |
| Molar mass | 286.749 g/mol |
| Appearance | yellow crystals |
| Density | 4.67 g/cm3, solid |
| Melting point | 265 °C (509 °F; 538 K) |
| Boiling point | sublimes > 350 °C in vacuo |
| decomposes | |
| Structure | |
| orthorhombic | |
| Hazards | |
|
EU classification (DSD)
|
not listed |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Tungsten dichloride dioxide is the chemical compound with the formula WO2Cl2. It is a yellow-colored solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO2Cl2 is sensitive to moisture, undergoing hydrolysis.
WO2Cl2 is prepared by ligand redistribution reaction from tungsten trioxide and tungsten hexachloride:
Using a two-zone tube furnace, a vacuum-sealed tube containing these solids is heated to 350 °C. The yellow product sublimes to the cooler end of the reaction tube. No redox occurs in this process. An alternative route highlights the oxophilicity of tungsten:
This reaction, like the preceding one, proceeds via the intermediacy of WOCl4.
The compound is a polymer consisting of distorted octahedral W centres. The monomer is characterized by two short W-O distances, typical for a multiple W-O bond, and two long W-O distances more typical of a single or datice W-O bond.
Tungsten forms a number of oxyhalides including WOCl4, WOCl3, WOCl2. The corresponding bromides (WOBr4, WOBr3, WOBr2) are also known as is WO2I2.
WO2Cl2 is a Lewis acid, forming soluble adducts of the type WO2Cl2L2, where L is a donor ligand such as bipyridine and dimethoxyethane. Such complexes often cannot be prepared by depolymerization of the inorganic solid, but are generated in situ from WOCl4.
...
Wikipedia
