Tungsten trioxide
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Tungsten trioxide
|
|
| Other names
Tungstic anhydride
Tungsten(VI) oxide Tungstic oxide |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ECHA InfoCard | 100.013.848 |
|
PubChem CID
|
|
| RTECS number | YO7760000 |
|
|
|
|
| Properties | |
| WO3 | |
| Molar mass | 231.84 g/mol |
| Appearance | Canary yellow powder |
| Density | 7.16 g/cm3 |
| Melting point | 1,473 °C (2,683 °F; 1,746 K) |
| Boiling point | 1,700 °C (3,090 °F; 1,970 K) approximation |
| insoluble | |
| Solubility | slightly soluble in HF |
| −15.8·10−6 cm3/mol | |
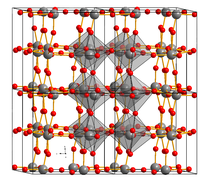
| Structure | |
| Monoclinic, mP32, Space group P121/c1, No 14 | |
| Octahedral (WVI) Trigonal planar (O2– ) |
|
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | External MSDS |
| Flash point | Non-flammable |
| Related compounds | |
|
Other anions
|
Tungsten trisulfide |
|
Other cations
|
Chromium trioxide Molybdenum trioxide |
|
Tungsten(III) oxide Tungsten(IV) oxide |
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Tungsten(VI) oxide, also known as tungsten trioxide or tungstic anhydride, WO3, is a chemical compound containing oxygen and the transition metal tungsten. It is obtained as an intermediate in the recovery of tungsten from its minerals. Tungsten ores are treated with alkalis to produce WO3. Further reaction with carbon or hydrogen gas reduces tungsten trioxide to the pure metal.
Tungsten(VI) oxide occurs naturally in the form of hydrates, which include minerals: tungstite WO3·H2O, meymacite WO3·2H2O and hydrotungstite (of the same composition as meymacite, however sometimes written as H2WO4). These minerals are rare to very rare secondary tungsten minerals.
Tungsten has a rich history dating back to its discovery during the 18th century. Peter Woulfe was the first to recognize a new element in the naturally occurring mineral wolframite. Tungsten was originally known as wolfram, explaining the choice of "W" for its elemental symbol. Swedish chemist Carl Wilhelm Scheele contributed to its discovery with his studies on the mineral scheelite.
In 1841, a chemist named Robert Oxland gave the first procedures for preparing tungsten trioxide and sodium tungstate. He was granted patents for his work soon after, and is considered to be the founder of systematic tungsten chemistry.
Tungsten trioxide can be prepared in several different ways. CaWO4, or scheelite, is allowed to react with HCl to produce tungstic acid, which decomposes to WO3 and water at high temperatures.
Another common way to synthesize WO3 is by calcination of ammonium paratungstate (APT) under oxidizing conditions:
...
Wikipedia
