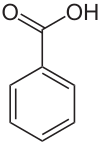
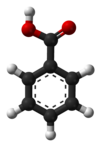
Benzoic (acid)
|
|
|||
 Benzoic acid crystals
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Benzoic acid
|
|||
|
Systematic IUPAC name
Benzenecarboxylic acid
|
|||
| Other names
Carboxybenzene; E210; Dracylic acid; Phenylmethanoic acid
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| 3DMet | B00053 | ||
| 636131 | |||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.562 | ||
| EC Number | 200-618-2 | ||
| E number | E210 (preservatives) | ||
| 2946 | |||
| KEGG | |||
| MeSH | benzoic+acid | ||
|
PubChem CID
|
|||
| RTECS number | DG0875000 | ||
| UNII | |||
|
|||
|
|||
| Properties | |||
| C7H6O2 | |||
| Molar mass | 122.12 g·mol−1 | ||
| Appearance | Colorless crystalline solid | ||
| Odor | faint, pleasant odor | ||
| Density | 1.2659 g/cm3 (15 °C) 1.0749 g/cm3 (130 °C) |
||
| Melting point | 122.41 °C (252.34 °F; 395.56 K) | ||
| Boiling point | 249.2 °C (480.6 °F; 522.3 K) 370 °C (698 °F; 643 K) decomposes |
||
| 1.7 g/L (0 °C) 2.7 g/L (18 °C) 3.44 g/L (25 °C) 5.51 g/L (40 °C) 21.45 g/L (75 °C) 56.31 g/L (100 °C) |
|||
| Solubility | soluble in acetone, benzene, CCl4, CHCl3, alcohol, ethyl ether, hexane, phenyls, liquid ammonia, acetates | ||
| Solubility in methanol | 30 g/100 g (-18 °C) 32.1 g/100 g (-13 °C) 71.5 g/100 g (23 °C) |
||
| Solubility in ethanol | 25.4 g/100 g (-18 °C) 47.1 g/100 g (15 °C) 52.4 g/100 g (19.2 °C) 55.9 g/100 g (23 °C) |
||
| Solubility in acetone | 54.2 g/100 g (20 °C) | ||
| Solubility in olive oil | 4.22 g/100 g (25 °C) | ||
| Solubility in 1,4-Dioxane | 55.3 g/100 g (25 °C) | ||
| log P | 1.87 | ||
| Vapor pressure | 0.16 Pa (25 °C) 0.19 kPa (100 °C) 22.6 kPa (200 °C) |
||
| Acidity (pKa) | 4.202 | ||
| -70.28·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.5397 (20 °C) 1.504 (132 °C) |
||
| Viscosity | 1.26 mPa (130 °C) | ||
| Structure | |||
| Monoclinic | |||
| planar | |||
| 1.72 D in Dioxane | |||
| Thermochemistry | |||
| 146.7 J/mol·K | |||
|
Std molar
entropy (S |
167.6 J/mol·K | ||
|
Std enthalpy of
formation (ΔfH |
-385.2 kJ/mol | ||
|
Std enthalpy of
combustion (ΔcH |
-3228 kJ/mol | ||
| Hazards | |||
| Main hazards | Irritant | ||
| Safety data sheet | JT Baker | ||
| GHS pictograms |
 
|
||
| GHS signal word | Danger | ||
| H318, H335 | |||
| P261, P280, P305+351+338 | |||
|
EU classification (DSD)
|
|||
| R-phrases | R37, R41 | ||
| S-phrases | S26, S39 | ||
| NFPA 704 | |||
| Flash point | 121.5 °C (250.7 °F; 394.6 K) | ||
| 571 °C (1,060 °F; 844 K) | |||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
1700 mg/kg (rat, oral) | ||
| Related compounds | |||
|
Related carboxylic acids
|
Hydroxybenzoic acids Aminobenzoic acids, Nitrobenzoic acids, Phenylacetic acid |
||
|
Related compounds
|
Benzaldehyde, Benzyl alcohol, Benzoyl chloride, Benzylamine, Benzamide |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Benzoic acid /bɛnˈzoʊ.ɪk/, C7H6O2 (or C6H5COOH), is a colorless crystalline solid and a simple aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only known source. Benzoic acid occurs naturally in many plants and it serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives and benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates /ˈbɛnzoʊ.eɪt/.
Benzoic acid was discovered in the sixteenth century. The dry distillation of gum benzoin was first described by Nostradamus (1556), and then by Alexius Pedemontanus (1560) and Blaise de Vigenère (1596).
Pioneer work in 1830 through a variety of experiences based on amygdalin, obtained from bitter almonds (the fruit of Prunus dulcis) oil by Pierre Robiquet and Antoine Boutron-Charlard, two French chemists, had produced benzaldehyde but they failed in working out a proper interpretation of the structure of amygdalin that would account for it, and thus missed the identification of the benzoyl radical C7H5O. This last step was achieved some few months later (1832) by Justus von Liebig and Friedrich Wöhler, who determined the composition of benzoic acid. These latter also investigated how hippuric acid is related to benzoic acid.
...
Wikipedia