Rimonabant
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Undetermined |
| Protein binding | Nearly 100% |
| Metabolism | Hepatic, CYP3A4 involved |
| Biological half-life | Variable: 6 to 9 days with normal BMI 16 days if BMI >30 |
| Excretion | Fecal (86%) and renal (3%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.233.193 |
| Chemical and physical data | |
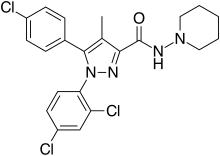
| Formula | C22H21Cl3N4O |
| Molar mass | 463.79 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Rimonabant (also known as SR141716; trade names Acomplia, Zimulti) is an anorectic antiobesity drug that has been withdrawn from the market due to potentially serious side effects. It was approved for use in Europe and other countries, but never approved in the United States. Rimonabant is an inverse agonist for the cannabinoid receptor CB1. It has also been shown to be a μ-opioid receptor antagonist (possibly the contributing factor in its reported dysphoric qualities). Its main effect is reduction in appetite.
Rimonabant was the first selective CB1 receptor blocker to be approved for use anywhere in the world. In Europe, it was indicated for use in conjunction with diet and exercise for patients with a body mass index (BMI) greater than 30 kg/m², or patients with a BMI greater than 27 kg/m² with associated risk factors, such as type 2 diabetes or dyslipidaemia. In the UK, it was available beginning in July 2006. As of 2008, the drug was available in 56 countries.
On 21 June 2006, the European Commission approved the sale of rimonabant in the then-25-member European Union as a prescription drug. Pharmaceutical company Sanofi-Aventis announced rimonabant would be launched in the United Kingdom. Sales began in July 2006. Sanofi-Aventis also projected that the drug would be sold shortly thereafter in Denmark, Ireland, Germany, Finland, and Norway. It was expected in Belgium and Sweden in 2007. Ordinary obesity would, according to official medical recommendations, not be enough to acquire the prescription in Sweden; there would be additional requirements concerning abnormal blood lipid levels.
...
Wikipedia
