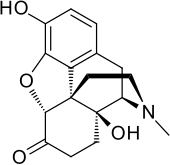
Oxymorphone
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Opana |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610022 |
| Pregnancy category |
|
| Dependence liability |
High |
| Routes of administration |
Oral, Intravenous, intramusucular, subcutaneous, rectal, intranasal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 10% Intranasal: 43% IV, IM: 100% |
| Protein binding | 10% |
| Metabolism | Hepatic (CYP3A4, glucuronidation) |
| Biological half-life | 7–9 hours |
| Excretion | Urine, feces |
| Identifiers | |
|
|
| Synonyms | 14-Hydroxydihydromorphinone |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.000.873 |
| Chemical and physical data | |
| Formula | C17H19NO4 |
| Molar mass | 301.33706 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Oxymorphone (brand names Opana, Numorphan, Numorphone), also known as 14-hydroxydihydromorphinone, is a powerful semi-synthetic opioid analgesic (painkiller) first developed in Germany in 1914, patented in the USA by Endo Pharmaceuticals in 1955 and introduced to the United States market in January 1959 and other countries around the same time.
The brand name Numorphan is derived by analogy to the Nucodan name for an oxycodone product (or vice versa) as well as Paramorphan/Paramorfan for dihydromorphine and Paracodin (dihydrocodeine). The only commercially available salt of oxymorphone in most of the world at this time is the hydrochloride, which has a free base conversion ratio of 0.891, and oxymorphone hydrochloride monohydrate has a factor of 0.85. It is a Schedule II controlled substance in the United States with an ACSCN of 9652. The 2013 DEA annual manufacturing quotas were 18 375 kilos for conversion (a number of drugs can be made from oxymorphone, both painkillers and opioid antagonists like naloxone) and 6875 kilos for direct manufacture of end-products.
The onset of analgesia after parenteral administration is about 5–10 minutes and after rectal administration, 15–30 minutes. The duration of analgesia is 3–4 hours for immediate-release tablets and 12 hours for extended-release tablets. Oxymorphone is also a minor metabolite of oxycodone, which is formed by CYP2D6-mediated O-demethylation.
Oxymorphone is indicated for the relief of moderate to severe pain and also as a preoperative medication to alleviate apprehension, maintain anaesthesia and as an obstetric analgesic. Additionally, it can be used for the alleviation of pain in patients with dyspnoea associated with acute left ventricular failure and pulmonary oedema (Brit.), or edema (Amer.). It is practically devoid of antitussive activity.
...
Wikipedia
