Neodymium(III) chloride
|
|
|||
| Names | |||
|---|---|---|---|
| Other names
Neodymium trichloride
|
|||
| Identifiers | |||
|
|||
|
3D model (Jmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.030.016 | ||
|
PubChem CID
|
|||
| UNII | |||
|
|||
|
|||
| Properties | |||
| NdCl3, NdCl3·6H2O (hydrate) |
|||
| Molar mass | 250.598 g/mol | ||
| Appearance | mauve-colored powder hygroscopic |
||
| Density | 4.13 g/cm3 (2.282 for hydrate) | ||
| Melting point | 758 °C (1,396 °F; 1,031 K) | ||
| Boiling point | 1,600 °C (2,910 °F; 1,870 K) | ||
| 0.967 kg/L at 13 °C | |||
| Solubility in ethanol | 0.445 kg/L | ||
| Structure | |||
| hexagonal (UCl3 type), hP8 | |||
| P63/m, No. 176 | |||
| Tricapped trigonal prismatic (nine-coordinate) |
|||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| Related compounds | |||
|
Other anions
|
Neodymium(III) bromide Neodymium(III) oxide |
||
|
Other cations
|
LaCl3, SmCl3, PrCl3, EuCl3, CeCl3, GdCl3, TbCl3, Promethium(III) chloride | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).
NdCl3 is a mauve colored hygroscopic solid whose color changes to purple upon absorption of atmospheric water. The resulting hydrate, like many other neodymium salts, has the interesting property that it appears different colors under fluorescent light- In the chloride's case, light yellow (see picture). The color difference is likely due to the charge transfer converting the Nd3+ ions into the Nd2+ state; the orange absorption band of Nd3+ decreases and the green Nd2+ absorption increases producing the yellowish color.
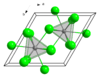
The anhydrous NdCl3 features Nd in a nine-coordinate tricapped trigonal prismatic geometry and crystallizes with the UCl3 structure. This hexagonal structure is common for many halogenated lanthanides and actinides such as LaCl3, LaBr3, SmCl3, PrCl3, EuCl3, CeCl3, CeBr3, GdCl3, AmCl3 and TbCl3 but not for YbCl3 and LuCl3.
...
Wikipedia