Samarium(III) chloride
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Samarium(III) chloride
|
|
| Identifiers | |
|
10361-82-7 (anhydrous) 13465-55-9 (hexahydrate) |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
55428 |
| ECHA InfoCard | 100.030.712 |
| UNII |
5J4QGH7J16 |
|
|
|
|
| Properties | |
| SmCl3 | |
| Molar mass | 256.76 g/mol (anhydrous) 364.80 g/mol (hexahydrate) |
| Appearance | pale yellow solid (anhydrous)
cream-coloured solid (hexahydrate) |
| Density | 4.46 g/cm3 (anhydrous)
2.383 g/cm3 (hexahydrate) |
| Melting point | 682 °C (1,260 °F; 955 K) |
| Boiling point | decomposes |
| 92.4 g/100 mL (10 °C) | |
| Structure | |
| hexagonal, hP8 | |
| P63/m, No. 176 | |
| Tricapped trigonal prismatic (nine-coordinate) |
|
| Hazards | |
| Main hazards | Irritant |
| Related compounds | |
|
Other anions
|
Samarium(III) oxide |
|
Other cations
|
Promethium(III) chloride, Europium(III) chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
cream-coloured solid (hexahydrate)
2.383 g/cm3 (hexahydrate)
Samarium(III) chloride, also known as samarium trichloride, is an inorganic compound of samarium and chloride. It is a pale yellow solid that rapidly absorbs water to form a hexahydrate, SmCl3.6H2O. The compound has few practical applications but is used in laboratories for research on new compounds of samarium.
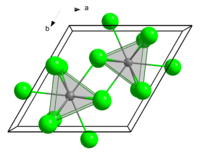
Like several related chlorides of the lanthanides and actinides, SmCl3 crystallises in the UCl3 motif. The Sm3+ centres are nine-coordinate, occupying trigonal prismatic sites with additional chloride ligands occupying the three square faces.
SmCl3 is prepared by the "ammonium chloride" route, which involves the initial synthesis of (NH4)2[SmCl5]. This material can be prepared from the common starting materials at reaction temperatures of 230 °C from samarium oxide:
The pentachloride is then heated to 350-400 °C resulting in evolution of ammonium chloride and leaving a residue of the anhydrous trichloride:
It can also be prepared from samarium metal and hydrogen chloride.
Aqueous solutions of samarium(III) chloride can be prepared by dissolving metallic samarium or samarium carbonate in hydrochloric acid.
Samarium(III) chloride is a moderately strong Lewis acid, which ranks as "hard" according to the HSAB concept. Aqueous solutions of samarium chloride can be used to prepare samarium trifluoride:
Samarium(III) chloride is used for the preparation of samarium metal, which has a variety of uses, notably in magnets. Anhydrous SmCl3 is mixed with sodium chloride or calcium chloride to give a low melting point eutectic mixture. Electrolysis of this molten salt solution gives the free metal.
...
Wikipedia
