Praseodymium(III) chloride
 |
|||
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Praseodymium(III) chloride
|
|||
| Other names
Praseodymium chloride; praseodymium trichloride
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ECHA InfoCard | 100.030.710 | ||
|
PubChem CID
|
|||
| UNII | |||
|
|||
|
|||
| Properties | |||
| PrCl3 | |||
| Molar mass | 247.24 g/mol (anhydrous) 373.77 g/mol (heptahydrate) |
||
| Appearance | blue-green solid (anhydrous) light green solid (heptahydrate) |
||
| Density | 4.02 g/cm3 (anhydrous) 2.250 g/cm3 (heptahydrate) |
||
| Melting point | 786 °C (1,447 °F; 1,059 K) | ||
| Boiling point | 1,710 °C (3,110 °F; 1,980 K) | ||
| 104.0 g/100 ml (13 °C) | |||
| +44.5·10−6 cm3/mol | |||
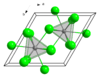
| Structure | |||
| hexagonal (UCl3 type), hP8 | |||
| P63/m, No. 176 | |||
| Tricapped trigonal prismatic (nine-coordinate) |
|||
| Hazards | |||
| Main hazards | Irritant | ||
| Related compounds | |||
|
Other anions
|
Praseodymium(III) oxide, Praseodymium(III) fluoride Praseodymium bromide praseodymium iodide |
||
|
Other cations
|
Cerium(III) chloride Neodymium(III) chloride |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Praseodymium(III) chloride is the inorganic compound with the formula PrCl3. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green heptahydrate.
Praseodymium(III) chloride is prepared by treating praseodymium metal and hydrogen chloride:
It is usually purified by vacuum sublimation.
Hydrated salts of praseodymium(III) chloride can be prepared by treatment of either praseodymium metal or praseodymium(III) carbonate with hydrochloric acid:
PrCl3∙7H2O is a hygroscopic substance, that will not crystallise from the mother liquor unless it is left to dry in a dessiccator. Anhydrous PrCl3 can be made by thermal dehydration of the hydrate at 400 °C in the presence of ammonium chloride. Alternatively the hydrate can be dehydrated using thionyl chloride.
Praseodymium(III) chloride is Lewis acidic, classified as "hard" according to the HSAB concept. Rapid heating of the hydrate may cause small amounts of hydrolysis. PrCl3 forms a stable Lewis acid-base complex K2PrCl5 by reaction with potassium chloride; this compound shows interesting optical and magnetic properties.
Aqueous solutions of praseodymium(III) chloride can be used to prepare insoluble praseodymium(III) compounds. For example, praseodymium(III) phosphate and praseodymium(III) fluoride can be prepared by reaction with potassium phosphate and sodium fluoride, respectively:
...
Wikipedia