Uranium trichloride
 |
|
| Names | |
|---|---|
|
IUPAC name
Uranium(III) chloride
|
|
| Other names
Uranium chloride
Uranium trichloride Hypouranous chloride |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| Cl3U | |
| Molar mass | 344.38 g·mol−1 |
| Appearance | Green crystalline solid |
| Density | 5.500 g/cm3, liquid |
| Melting point | 837 °C (1,539 °F; 1,110 K) |
| Boiling point | 1,657 °C (3,015 °F; 1,930 K) |
| Soluble | |
| Structure | |
| Hybridisation | Tricapped trigonal prismatic |
| Hazards | |
| Flash point | Non-flammable |
| Non-flammable | |
| Related compounds | |
|
Related compounds
|
Uranium(IV) chloride, Uranium(V) chloride, Uranium(VI) chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Uranium(III) chloride, UCl3, is a chemical compound that contains the earth metal uranium and chlorine. UCl3 is used mostly to reprocess spent nuclear fuel. Uranium(III) chloride is synthesized in various ways from uranium(IV) chloride; however, UCl3 is less stable than UCl4.
There are two ways to synthesize uranium(III) chloride. The following processes describe how to produce uranium(III) chloride.
(1) In a mixture of NaCl-KCl at 670–710 °C, add uranium tetrachloride with uranium metal.
(2) Heat uranium(IV) chloride in hydrogen gas.
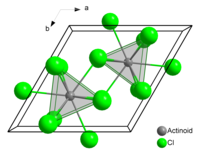
In solid uranium(III) chloride each uranium atom has nine chlorine atoms as near neighbours, at approximately the same distance, in a tricapped trigonal prismatic configuration.
Uranium(III) chloride is a green crystalline solid at room temperature. UCl3 melts at 837 °C and boils at 1657 °C. Uranium(III) chloride has a density of 5500 kg/m3 or 5.500 g/cm3.
Its composition by weight:
Its formal oxidative states:
Uranium(III) chloride is very soluble in water and is also very hygroscopic. UCl3 is more stable in a solution of hydrochloric acid.
Uranium(III) chloride is used in reactions with tetrahydrofuran (THF) and sodium methylcyclopentadiene to prepare various uranium metallocene complexes.
Uranium(III) chloride is used as a catalyst during reactions between lithium aluminium hydride (LiAlH4) and olefins to produce alkyl aluminate compounds.
The molten form of uranium(III) chloride is a typical compound in pyrochemical processes as it is important in the reprocessing of spent nuclear fuels. UCl3 is usually the form that uranium takes as spent fuel in electrorefining processes.
There are three hydrates of uranium(III) chloride:
Each are synthesized by the reduction of uranium(IV) chloride in methylcyanide (acetonitrile), with specific amounts of water and propionic acid.
...
Wikipedia
