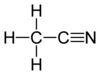
Acetonitrile
|
|
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Acetonitrile
|
|||
|
Systematic IUPAC name
Ethanenitrile
|
|||
Other names
|
|||
| Identifiers | |||
|
75-05-8 |
|||
| 3D model (Jmol) | Interactive image | ||
| 741857 | |||
| ChEBI |
CHEBI:38472 |
||
| ChEMBL |
ChEMBL45211 |
||
| ChemSpider |
6102 |
||
| ECHA InfoCard | 100.000.760 | ||
| EC Number | 200-835-2 | ||
| 895 | |||
| MeSH | acetonitrile | ||
| PubChem | 6342 | ||
| RTECS number | AL7700000 | ||
| UNII |
Z072SB282N |
||
| UN number | 1648 | ||
|
|||
|
|||
| Properties | |||
| C2H3N | |||
| Molar mass | 41.05 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.786 g/cm3 | ||
| Melting point | −46 to −44 °C; −51 to −47 °F; 227 to 229 K | ||
| Boiling point | 81.3 to 82.1 °C; 178.2 to 179.7 °F; 354.4 to 355.2 K | ||
| Miscible | |||
| log P | −0.334 | ||
| Vapor pressure | 9.71 kPa (at 20.0 °C) | ||
|
Henry's law
constant (kH) |
530 μmol/(Pa·kg) | ||
| Acidity (pKa) | 25 | ||
| Basicity (pKb) | −11 | ||
| UV-vis (λmax) | 195 nm | ||
| Absorbance | ≤0.10 | ||
| −28.0×10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.344 | ||
| Thermochemistry | |||
| 91.69 J/(K·mol) | |||
|
Std molar
entropy (S |
149.62 J/(K·mol) | ||
|
Std enthalpy of
formation (ΔfH |
40.16–40.96 kJ/mol | ||
|
Std enthalpy of
combustion (ΔcH |
−1256.03 – −1256.63 kJ/mol | ||
| Hazards | |||
| Safety data sheet | See: data page | ||
| GHS pictograms |
 
|
||
| GHS signal word | DANGER | ||
| H225, H302, H312, H319, H332 | |||
| P210, P280, P305+351+338 | |||
|
EU classification (DSD)
|
|
||
| R-phrases | R11, R20/21/22, R36 | ||
| S-phrases | (S1/2), S16, S36/37 | ||
| NFPA 704 | |||
| Flash point | 2.0 °C (35.6 °F; 275.1 K) | ||
| 523.0 °C (973.4 °F; 796.1 K) | |||
| Explosive limits | 4.4–16.0% | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
|
||
|
LC50 (median concentration)
|
5655 ppm (guinea pig, 4 hr) 2828 ppm (rabbit, 4 hr) 53,000 ppm (rat, 30 min) 7500 ppm (rat, 8 hr) 2693 ppm (mouse, 1 hr) |
||
|
LCLo (lowest published)
|
16,000 ppm (dog, 4 hr) | ||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 40 ppm (70 mg/m3) | ||
|
REL (Recommended)
|
TWA 20 ppm (34 mg/m3) | ||
|
IDLH (Immediate danger)
|
500 ppm | ||
| Related compounds | |||
|
Related alkanenitriles
|
|||
|
Related compounds
|
DBNPA | ||
| Supplementary data page | |||
|
Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Acetonitrile is the chemical compound with the formula CH
3CN. This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene.
In the laboratory, it is used as a medium-polarity solvent that is miscible with water and a range of organic solvents, but not saturated hydrocarbons. It has a convenient liquid range and a high dielectric constant of 38.8. With a dipole moment of 3.92 D, acetonitrile dissolves a wide range of ionic and nonpolar compounds and is useful as a mobile phase in HPLC and LC–MS. The N≡C−C skeleton is linear with a short C≡N distance of 1.16 Å.
Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas.
Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separating tower to separate the butadiene.
...
Wikipedia