Lutetium(III) chloride
 |
|
| Names | |
|---|---|
|
IUPAC name
Lutetium(III) chloride
|
|
| Other names
Lutetium chloride, lutetium trichloride
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.205 |
|
PubChem CID
|
|
| RTECS number | OK8400000 |
| UNII | |
|
|
|
|
| Properties | |
| LuCl3 | |
| Molar mass | 281.325 g/mol |
| Appearance | colorless or white monoclinic crystals |
| Density | 3.98 g/cm3 |
| Melting point | 905 °C (1,661 °F; 1,178 K) |
| Boiling point | sublimes above 750°C |
| soluble | |
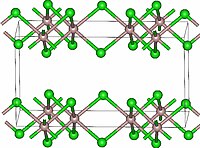
| Structure | |
| Monoclinic, mS16 | |
| C2/m, No. 12 | |
| Hazards | |
| Main hazards | Xi (Irritant) |
| R-phrases | R36/37/38 |
| S-phrases | S26, S36 |
| NFPA 704 | |
| Related compounds | |
|
Other anions
|
Lutetium(III) oxide |
|
Other cations
|
Ytterbium(III) chloride Scandium(III) chloride Yttrium(III) chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals. Lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions.
Pure lutetium metal can be produced from lutetium(III) chloride by heating it together with elemental calcium:
...
Wikipedia

