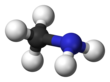
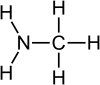
Monomethylamine
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Methanamine
|
|||
Other names
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| 3DMet | B00060 | ||
| Abbreviations | MMA | ||
| 741851 | |||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.746 | ||
| EC Number | 200-820-0 | ||
| 145 | |||
| KEGG | |||
| MeSH | methylamine | ||
|
PubChem CID
|
|||
| RTECS number | PF6300000 | ||
| UN number | 1061 | ||
|
|||
|
|||
| Properties | |||
| CH5N | |||
| Molar mass | 31.06 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Fishy, ammoniacal | ||
| Density | 656.2 kg m−3 (at 25 °C) | ||
| Melting point | −93.10 °C; −135.58 °F; 180.05 K | ||
| Boiling point | −6.6 to −6.0 °C; 20.0 to 21.1 °F; 266.5 to 267.1 K | ||
| 1.08 kg L−1 (at 20 °C) | |||
| log P | −0.472 | ||
| Vapor pressure | 186.10 kPa (at 20 °C) | ||
|
Henry's law
constant (kH) |
1.4 mmol Pa−1 kg−1 | ||
| Basicity (pKb) | 3.36 | ||
| -27.0·10−6 cm3/mol | |||
| Viscosity | 230 μPa s (at 0 °C) | ||
| 1.31 D | |||
| Thermochemistry | |||
|
Std enthalpy of
formation (ΔfH |
−23.5 kJ mol−1 | ||
| Hazards | |||
| Safety data sheet | emdchemicals.com | ||
| GHS pictograms |
  
|
||
| GHS signal word | DANGER | ||
| H220, H315, H318, H332, H335 | |||
| P210, P261, P280, P305+351+338, P410+403 | |||
| NFPA 704 | |||
| Flash point | −10 °C; 14 °F; 263 K (liquid, gas is extremely flammable) | ||
| 430 °C (806 °F; 703 K) | |||
| Explosive limits | 4.9–20.7% | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
100 mg kg−1(oral, rat) | ||
|
LC50 (median concentration)
|
1860 ppm (mouse, 2 hr) | ||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 10 ppm (12 mg/m3) | ||
|
REL (Recommended)
|
TWA 10 ppm (12 mg/m3) | ||
|
IDLH (Immediate danger)
|
100 ppm | ||
| Related compounds | |||
|
Related alkanamines
|
ethylamine, dimethylamine, trimethylamine | ||
|
Related compounds
|
ammonia | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Methylamine is an organic compound with a formula of CH3NH2. This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, tetrahydrofuran, or water, or as the anhydrous gas in pressurized metal containers. Industrially, methylamine is transported in its anhydrous form in pressurized railcars and tank trailers. It has a strong odor similar to fish. Methylamine is used as a building block for the synthesis of many other commercially available compounds.
Methylamine is prepared commercially by the reaction of ammonia with methanol in the presence of an aluminosilicate catalyst. Dimethylamine and trimethylamine are co-produced; the reaction kinetics and reactant ratios determine the ratio of the three products. The product most favoured by the reaction kinetics is trimethylamine.
In this way, an estimated 115,000 tons were produced in 2005.
Methylamine was first prepared in 1849 by Charles-Adolphe Wurtz via the hydrolysis of methyl isocyanate and related compounds. An example of this process includes the use of the Hofmann rearrangement, to yield methylamine from acetamide and bromine gas.
In the laboratory, methylamine hydrochloride is readily prepared by various other methods. One method entails treating formaldehyde with ammonium chloride.
...
Wikipedia