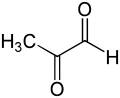
Methylglyoxal
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
2-Oxopropanal
|
|||
| Other names
Pyruvaldehyde
|
|||
| Identifiers | |||
|
(hydrate: 1186-47-6) 78-98-8 (hydrate: 1186-47-6) |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:17158 |
||
| ChEMBL |
ChEMBL170721 |
||
| ChemSpider |
857 |
||
| DrugBank |
DB03587 |
||
| ECHA InfoCard | 100.001.059 | ||
| 6303 | |||
| KEGG |
C00546 |
||
| MeSH | Methylglyoxal | ||
| PubChem | 880 | ||
| UNII |
722KLD7415 |
||
|
|||
|
|||
| Properties | |||
| C3H4O2 | |||
| Molar mass | 72.06 g·mol−1 | ||
| Appearance | Yellow liquid | ||
| Density | 1.046 g/cm3 | ||
| Boiling point | 72 °C (162 °F; 345 K) | ||
| Related compounds | |||
|
glyoxal propionaldehyde propanedial acetone diacetyl acetylacetone |
|||
|
Related compounds
|
glyoxylic acid pyruvic acid acetoacetic acid |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Methylglyoxal, also called pyruvaldehyde or 2-oxopropanal, is the organic compound with the formula CH3C(O)CHO. Gaseous methylglyoxal has two carbonyl groups, an aldehyde and a ketone but in the presence of water, it exists as hydrates and oligomers. It is a reduced derivative of pyruvic acid.
Methylglyoxal is produced industrially by degradation of carbohydrates using overexpressed methylglyoxal synthase.
In organisms, methylglyoxal is formed as a side-product of several metabolic pathways. It may form from 3-aminoacetone, which is an intermediate of threonine catabolism, as well as through lipid peroxidation. However, the most important source is glycolysis. Here, methylglyoxal arises from nonenzymatic phosphate elimination from glyceraldehyde phosphate and dihydroxyacetone phosphate, two intermediates of glycolysis. Since methylglyoxal is highly cytotoxic, the body developed several detoxification mechanisms. One of these is the glyoxalase system. Methylglyoxal reacts with glutathione to form a hemithioacetal. This is converted into S-D-lactoyl-glutathione by glyoxalase I, and then further metabolized into D-lactate by glyoxalase II.
The proximate and ultimate causes for biological methylglyoxal production remain unknown, but it may be involved in the formation of advanced glycation endproducts (AGEs). In this process, methylglyoxal reacts with free amino groups of lysine and arginine and with thiol groups of cysteine forming AGEs. Recent research has identified heat shock protein 27 (Hsp27) as a specific target of posttranslational modification by methylglyoxal in human metastatic melanoma cells.
...
Wikipedia