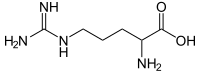
Arginine
 |
|
 |
|
| Names | |
|---|---|
| Other names
2-Amino-5-guanidinopentanoic acid
|
|
| Identifiers | |
|
7200-25-1 157-06-2 R 74-79-3 S |
|
| 3D model (Jmol) |
Interactive image Interactive image |
| 3DMet | B01331 |
| 1725411, 1725412 R, 1725413 S | |
| ChEBI |
CHEBI:29016 |
| ChEMBL |
ChEMBL212301 ChEMBL1485 |
| ChemSpider |
227 64224 R 6082 S |
| DrugBank |
DB00125 |
| ECHA InfoCard | 100.000.738 |
| EC Number | 230-571-3 |
| 364938 R | |
| 721 | |
| KEGG |
C02385 |
| MeSH | Arginine |
| PubChem |
232 71070 R 6322 S |
| RTECS number | CF1934200 S |
| UNII |
94ZLA3W45F |
|
|
|
|
| Properties | |
| C6H14N4O2 | |
| Molar mass | 174.20 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| Melting point | 260 °C; 500 °F; 533 K |
| Boiling point | 368 °C (694 °F; 641 K) |
| 14.87 g/100 mL (20 °C) | |
| Solubility | slightly soluble in ethanol insoluble in ethyl ether |
| log P | −1.652 |
| Acidity (pKa) | 12.488 |
| Basicity (pKb) | 1.509 |
| Thermochemistry | |
| 232.8 J K−1 mol−1 (at 23.7 °C) | |
|
Std molar
entropy (S |
250.6 J K−1 mol−1 |
|
Std enthalpy of
formation (ΔfH |
−624.9–−622.3 kJ mol−1 |
|
Std enthalpy of
combustion (ΔcH |
−3.7396–−3.7370 MJ mol−1 |
| Pharmacology | |
| B05XB01 (WHO) S | |
| Hazards | |
| Safety data sheet |
See: data page sigma-aldrich |
| GHS pictograms |  |
| GHS signal word | WARNING |
| H319 | |
| P305+351+338 | |
|
EU classification (DSD)
|
|
| R-phrases | R36 |
| S-phrases | S26 |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
5110 mg/kg (rat, oral) |
| Related compounds | |
|
Related alkanoic acids
|
|
|
Related compounds
|
|
| Supplementary data page | |
|
Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Arginine (abbreviated as Arg or R) encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG is an α-amino acid that is used in the biosynthesis of proteins.
Arginine is classified as a semiessential or conditionally essential amino acid, depending on the developmental stage and health status of the individual. Preterm infants are unable to synthesize or create arginine internally, making the amino acid nutritionally essential for them. Most healthy people do not need to supplement with arginine because their body produces sufficient amounts.
Arginine was first isolated from a lupin seedling extract in 1886 by the German chemist Ernst Schultze. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain of a 3-carbon aliphatic straight chain capped by a complex guanidinium, classifying it as a charged (at physiological pH), aliphatic amino acid.
A conditionally essential amino acid is one that may be required depending on the health status or life cycle of the individual. Arginine is one such conditionally essential amino acid. The biosynthetic pathway, however, does not produce sufficient arginine, and some must still be consumed through diet. Individuals with poor nutrition or certain physical conditions may be advised to increase their intake of foods containing arginine. Arginine is found in a wide variety of foods, including:
Arginine is synthesized from citrulline in arginine and proline metabolism by the sequential action of the cytosolic enzymes argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL). In terms of energy, this is costly, as the synthesis of each molecule of argininosuccinate requires hydrolysis of adenosine triphosphate (ATP) to adenosine monophosphate (AMP), i.e., two ATP equivalents. In essence, taking an excess of arginine gives more energy by saving ATPs that can be used elsewhere.
...
Wikipedia
