Guanidinium
|
|
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC names
Guanidine
Iminomethanediamine |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| 506044 | |||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.656 | ||
| EC Number | 204-021-8 | ||
| 100679 | |||
| MeSH | Guanidine | ||
|
PubChem CID
|
|||
| UNII | |||
|
|||
|
|||
| Properties | |||
| CH5N3 | |||
| Molar mass | 59.07 g·mol−1 | ||
| Melting point | 50 °C (122 °F; 323 K) | ||
| log P | −1.251 | ||
| Acidity (pKa) | 13.6 | ||
| Thermochemistry | |||
|
Std enthalpy of
formation (ΔfH |
−57 – −55 kJ mol−1 | ||
|
Std enthalpy of
combustion (ΔcH |
−1.0511 – −1.0531 MJ mol−1 | ||
| Pharmacology | |||
| Pharmacokinetics: | |||
| 7–8 hours | |||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
475 mg/kg (oral, rat) | ||
| Related compounds | |||
|
Related compounds
|
|||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
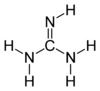
Guanidine is the compound with the formula HNC(NH
2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine as a normal product of protein metabolism. Guanidine is the functional group on the side chain of arginine.
Guanidine can be thought of as a nitrogenous analogue of carbonic acid. That is, the C=O group in carbonic acid is replaced by a C=NH group, and each OH is replaced by a NH
2 group. A detailed crystallographic analysis of guanidine was elucidated 148 years after its first synthesis, despite the simplicity of the molecule. In 2013, the positions of the hydrogen atoms and their displacement parameters were accurately determined using single-crystal neutron diffraction.
Guanidine can be obtained from natural sources, being first isolated by Adolph Strecker via the degradation of guanine.
The compound was first synthesized in 1861 by the oxidative degradation of an aromatic natural product, guanine, isolated from Peruvian guano. The commercial route involves a two step process starting with the reaction of dicyandiamide with ammonium salts. Via the intermediacy of biguanidine, this ammonolysis step affords salts of the guanidinium cation (see below). In the second step, the salt is treated with base, such as sodium methoxide.
With a pKb of 0.4, guanidine is a strong base (a strength usually alternatively expressed as pKa = 13.6 for the guanidinium cation). In neutral water, it exists exclusively as guanidinium (C(NH
2)+
3). Most guanidine derivatives are in fact such salts.
...
Wikipedia