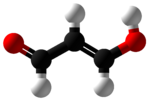
Propanedial
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
propanedial
|
|||
| Other names
Malonic aldehyde; Malonodialdehyde; Propanedial; 1,3-Propanedial ; Malonaldehyde
|
|||
| Identifiers | |||
|
542-78-9 |
|||
| 3D model (Jmol) | Interactive image | ||
| Abbreviations | MDA | ||
| ChemSpider |
10499 |
||
| KEGG |
C19440 |
||
| PubChem | 10964 | ||
|
|||
|
|||
| Properties | |||
| C3H4O2 | |||
| Molar mass | 72.06 g·mol−1 | ||
| Appearance | Needle-like solid | ||
| Density | 0.991 g/mL | ||
| Melting point | 72 °C (162 °F; 345 K) | ||
| Boiling point | 108 °C (226 °F; 381 K) | ||
| Hazards | |||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
none | ||
|
REL (Recommended)
|
Ca | ||
|
IDLH (Immediate danger)
|
Ca [N.D.] | ||
| Related compounds | |||
|
Related alkenals
|
Glucic acid |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Malondialdehyde (MDA) is the organic compound with the formula CH2(CHO)2. The structure of this species is more complex than this formula suggests. This reactive species occurs naturally and is a marker for oxidative stress.
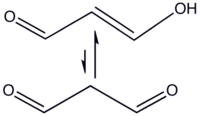
Malondialdehyde mainly exists in the enol form:
In organic solvents, the cis-isomer is favored, whereas in water the trans-isomer predominates.
Malondialdehyde is a highly reactive compound that is not typically observed in pure form. In the laboratory it can be generated in situ by hydrolysis of 1,1,3,3-tetramethoxypropane, which is commercially available. It is easily deprotonated to give the sodium salt of the enolate (m.p. 245 °C).
Malondialdehyde results from lipid peroxidation of polyunsaturated fatty acids. It is a prominent product in Thromboxane A2 synthesis wherein cyclooxygenase 1 or cycloxygenase 2 metabolizes arachidonic acid to prostaglandin H2 by platelets and a wide array of other cell types and tissues. This product is further metabolized by Thromboxane synthase to Thromboxane A2, 12-Hydroxyheptadecatrienoic acid, and malonyldialdehyde. Alternatively, it may rearrange non-enzymatically to a mixture of 8-cis and 8-trans isomers of 12-hydroxyeicosaheptaenoic acid plus malonyldialdehyde (see 12-Hydroxyheptadecatrienoic acid). The degree of lipid peroxidation can be estimated by the amount of malondialdehyde in tissues.
Reactive oxygen species degrade polyunsaturated lipids, forming malondialdehyde. This compound is a reactive aldehyde and is one of the many reactive electrophile species that cause toxic stress in cells and form covalent protein adducts referred to as advanced lipoxidation end-products (ALE), in analogy to advanced glycation end-products (AGE). The production of this aldehyde is used as a biomarker to measure the level of oxidative stress in an organism.
...
Wikipedia