Febuxostat
 |
|
| Clinical data | |
|---|---|
| Trade names | Uloric (US), Adenuric (Europe), others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609020 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
by mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~49% absorbed |
| Protein binding | ~99% to albumin |
| Metabolism | via CYP1A2, 2C8, 2C9, UGT1A1, 1A3, 1A9, 2B7 |
| Biological half-life | ~5-8 hours |
| Excretion | Urine (~49% mostly as metabolites, 3% as unchanged drug); feces (~45% mostly as metabolites, 12% as unchanged drug) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.207.329 |
| Chemical and physical data | |
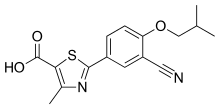
| Formula | C16H16N2O3S |
| Molar mass | 316.374 g/mol |
| 3D model (JSmol) | |
|
|
|
|
|
|
|
Febuxostat, sold under the brand names Uloric in the US and Adenuric in Europe, is a medication used in the treatment of chronic gout and hyperuricemia. It inhibits xanthine oxidase, thus reducing production of uric acid in the body.
Febuxostat was discovered by scientists at the Japanese pharmaceutical company Teijin in 1998. Teijin partnered the drug with TAP Pharmaceuticals in the US and Ipsen in Europe. Ipsen obtained marketing approval for febuxostat from the European Medicines Agency in April 2008, Takeda obtained FDA approval in February 2009, and Teijin obtained approval from the Japanese Pharmaceuticals and Medical Devices Agency in 2011.
Febuxostat is used to treat chronic gout and hyperuricemia.National Institute for Health and Clinical Excellence concluded that febuxostat is more effective than standard doses of allopurinol, but not more effective than higher doses of allopurinol.
Febuxostat is in the US pregnancy category C; there are no adequate and well-controlled studies in pregnant women.
The adverse effects associated with febuxostat therapy include nausea, diarrhea, arthralgia, headache, increased hepatic serum enzyme levels and rash.
Febuxostat is contraindicated with concomitant use of theophylline and chemotherapeutic agents, namely azathioprine and 6-mercaptopurine, because it could increase blood plasma concentrations of these drugs, and therefore their toxicity.
...
Wikipedia
