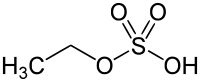
Ethyl sulfate
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Ethyl hydrogen sulfate
|
|
| Other names
Ethyl sulfate; Sulfovinic acid; Ethyl bisulfate; Ethoxysulfonic acid; Ethyl sulphate
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.963 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C2H6O4S | |
| Molar mass | 126.13 g·mol−1 |
| Density | 1.46 g/cm³ |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Ethyl sulfate (IUPAC name: ethyl hydrogen sulfate), also known as sulfovinic acid, is an organic chemical compound used as an intermediate in the production of ethanol from ethylene. It is the ethyl ester of sulfuric acid.
This substance was studied alongsideether for the first time by German alchemist August Siegmund Frobenius in 1730, subsequently by French chemists Fourcroy in 1797 and Gay-Lussac in 1815.Swiss scientist Nicolas-Théodore de Saussure also studied it in 1807. In 1827, French chemist and pharmacist Félix-Polydore Boullay (1806-1835) along with Jean-Baptiste André Dumas noted the role of ethyl sulfate in the preparation of diethyl ether from sulfuric acid and ethanol. Further studies by the German chemist Eilhard Mitscherlich and the Swedish chemist Jöns Berzelius suggested sulfuric acid was acting as a catalyst, this eventually led to the discovery of sulfovinic acid as an intermediate in the process. The advent of electrochemistry by Italian physicist Alessandro Volta and English chemist Humphry Davy in the 1800s confirmed ether and water were formed by the reaction of sub-stoichiometric amounts of sulfuric acid on ethanol and that sulfovinic acid was formed as an intermediate in the reaction.
...
Wikipedia
