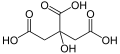
Citric acid
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
2-Hydroxypropane-1,2,3-tricarboxylic acid
|
|||
| Other names
Citric acid
|
|||
| Identifiers | |||
|
77-92-9 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:30769 |
||
| ChEMBL |
ChEMBL1261 |
||
| ChemSpider |
305 |
||
| DrugBank |
DB04272 |
||
| ECHA InfoCard | 100.000.973 | ||
| EC Number | 201-069-1 | ||
| E number | E330 (antioxidants, ...) | ||
| 2478 | |||
| KEGG |
D00037 |
||
| PubChem | 22230 (monohydrate) 311, 22230 (monohydrate) | ||
| RTECS number | GE7350000 | ||
| UNII |
XF417D3PSL |
||
|
|||
|
|||
| Properties | |||
| C6H8O7 | |||
| Molar mass | 192.12 g·mol−1 | ||
| Appearance | crystalline white solid | ||
| Odor | odorless | ||
| Density | 1.665 g/cm3 (anhydrous) 1.542 g/cm3 (18 °C, monohydrate) |
||
| Melting point | 156 °C (313 °F; 429 K) | ||
| Boiling point | 310 °C (590 °F; 583 K) decomposes from 175 °C | ||
| 117.43 g/100 mL (10 °C) 147.76 g/100 mL (20 °C) 180.89 g/100 mL (30 °C) 220.19 g/100 mL (40 °C) 382.48 g/100 mL (80 °C) 547.79 g/100 mL (100 °C) |
|||
| Solubility | soluble in alcohol, ether, ethyl acetate, DMSO insoluble in C6H6, CHCl3, CS2, toluene |
||
| Solubility in ethanol | 62 g/100 g (25 °C) | ||
| Solubility in amyl acetate | 4.41 g/100 g (25 °C) | ||
| Solubility in diethyl ether | 1.05 g/100 g (25 °C) | ||
| Solubility in 1,4-Dioxane | 35.9 g/100 g (25 °C) | ||
| log P | −1.64 | ||
| Acidity (pKa) | pKa1 = 3.13 pKa2 = 4.76 pKa3 = 6.39, 6.40 |
||
|
Refractive index (nD)
|
1.493–1.509 (20 °C) 1.46 (150 °C) |
||
| Viscosity | 6.5 cP (50% aq. sol.) | ||
| Structure | |||
| Monoclinic | |||
| Thermochemistry | |||
| 226.51 J/(mol·K) (26.85 °C) | |||
|
Std molar
entropy (S |
252.1 J/(mol·K) | ||
|
Std enthalpy of
formation (ΔfH |
−1548.8 kJ/mol | ||
|
Std enthalpy of
combustion (ΔcH |
−1960.6 kJ/mol −1972.34 kJ/mol (monohydrate) |
||
| Pharmacology | |||
| A09AB04 (WHO) | |||
| Hazards | |||
| Main hazards | skin and eye irritant | ||
| Safety data sheet | HMDB | ||
| GHS pictograms |  |
||
| GHS signal word | Warning | ||
| H319 | |||
| P305+351+338 | |||
|
EU classification (DSD)
|
|
||
| R-phrases | R34, R36/37/38, R41 | ||
| S-phrases | S24/25, S26, S36/37/39, S45 | ||
| NFPA 704 | |||
| Flash point | 155 °C (311 °F; 428 K) | ||
| 345 °C (653 °F; 618 K) | |||
| Explosive limits | 8% | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
3000 mg/kg (rats, oral) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Citric acid is a weak organic tricarboxylic acid having the chemical formula C6H8O7. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
More than a million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring and chelating agent.
A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate ion is written as C6H5O73− or C3H5O(COO)33−.
Citric acid exists in greater than trace amounts in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L in the juices). The concentrations of citric acid in citrus fruits range from 0.005 mol/L for oranges and grapefruits to 0.30 mol/L in lemons and limes. Within species, these values vary depending on the cultivar and the circumstances in which the fruit was grown.
Industrial-scale citric acid production first began in 1890 based on the Italian citrus fruit industry, where the juice was treated with hydrated lime (calcium hydroxide) to precipitate calcium citrate, which was isolated and converted back to the acid using diluted sulfuric acid. In 1893, C. Wehmer discovered Penicillium mold could produce citric acid from sugar. However, microbial production of citric acid did not become industrially important until World War I disrupted Italian citrus exports.
...
Wikipedia