Calcium citrate
 Calcium citrate tetrahydrate
|
|
| Names | |
|---|---|
|
IUPAC name
2-hydroxy-1,2,3-propane-tricarboxylic acid calcium salt (2:3)
|
|
| Other names
E333, tricalcium dicitrate
|
|
| Identifiers | |
|
813-94-5 5785-44-4 (tetrahydrate) |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
12584 |
| EC Number | 212-391-7 |
| E number | E333 (antioxidants, ...) |
| PubChem | 13136 |
|
|
|
|
| Properties | |
| Ca3(C6H5O7)2 | |
| Molar mass | 498.4334 g/mol (anhydrous) 570.4945 g/mol (tetrahydrate) |
| Appearance | White powder |
| Odor | odorless |
| Density | 1.63 g/cm3 (anhydrous) 2.00 g/cm3 (tetrahydrate) |
| Melting point | Decomposes |
| Boiling point | Decomposes |
| 0.85 g/L (18 °C) 0.95 g/L (25 °C) |
|
| Solubility | insoluble in alcohol |
| Structure | |
| Triclinic (tetrahydrate) | |
| P1, No. 2 | |
|
a = 0.59466(4) nm, b = 1.02247(8) nm, c = 1.66496(13) nm
α = 72.213(7)°, β = 79.718(7)°, γ = 89.791(6)°
|
|
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | External MSDS |
| NFPA 704 | |
| Related compounds | |
|
Other cations
|
Sodium citrate |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Calcium citrate is the calcium salt of citric acid. It is commonly used as a food additive (E333), usually as a preservative, but sometimes for flavor. In this sense, it is similar to sodium citrate. Calcium citrate is also found in some dietary calcium supplements (e.g. Citracal). Calcium makes up 24.1% of calcium citrate (anhydrous) and 21.1% of calcium citrate (tetrahydrate) by mass. The tetrahydrate occurs in nature as the mineral Earlandite.
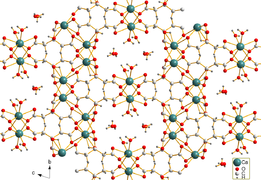
Calcium citrate is sparingly soluble in water. Needle-shaped crystals of tricalcium dicitrate tetrahydrate [Ca3(C6H5O7)2(H2O)2]·2H2O were obtained by hydrothermal synthesis. The crystal structure comprises a three-dimensional network in which eightfold coordinated Ca2+ cations are linked by citrate anions and hydrogen bonds between two non-coordinating crystal water molecules and two coordinating water molecules.
Calcium citrate is an intermediate in the isolation of citric acid from the fermentation process by which citric acid is produced industrially. The citric acid in the broth solution is neutralized by calcium hydroxide, precipitating insoluble calcium citrate. This is then filtered off from the rest of the broth and washed to give clean calcium citrate.
The calcium citrate thus produced may be sold as-is, or it may be converted to citric acid using dilute sulfuric acid.
In many individuals, bioavailability of calcium citrate is found to be equal to that of the cheaper calcium carbonate. However, alterations to the digestive tract may change how calcium is digested and absorbed. Unlike calcium carbonate, which is basic and neutralizes stomach acid, calcium citrate has no effect on stomach acid. Calcium carbonate is harder to digest than calcium citrate, and calcium carbonate carries a risk of "acid rebound" (the stomach overcompensates by producing more acid), so individuals who are sensitive to antacids or who have difficulty producing adequate stomach acid may choose calcium citrate over calcium carbonate for supplementation. According to recent research into calcium absorption after gastric bypass surgery, calcium citrate may have improved bioavailability over calcium carbonate in Roux-en-Y gastric bypass patients who are taking calcium citrate as a dietary supplement after surgery. This is mainly due to the changes related to where calcium absorption occurs in the digestive tract of these individuals.
...
Wikipedia

