Alvocidib
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
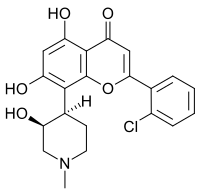
2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methyl-4-piperidinyl]-4-chromenone
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
| MeSH | Flavopiridol |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C21H20ClNO5 | |
| Molar mass | 401.8402 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Alvocidib (INN; also known as Flavopiridol or HMR-1275) is a flavonoid alkaloid CDK9 kinase inhibitor under clinical development for the treatment of acute myeloid leukemia, by Tolero Pharmaceuticals, Inc. It has been studied also for the treatment of arthritis and atherosclerotic plaque formation The target of Flavopiridol is the positive transcription elongation factor P-TEFb. Treatment of cells with Flavopiridol leads to inhibition of P-TEFb and the loss of mRNA production.
Flavone inhibitor of cyclin-dependent kinases; the (-)-cis form induces apoptosis in certain tumor cells.
Tetrahydropyridine (1) is obtainable by reacting N-methyl-4-piperidone with 1,3,5-trimethoxybenzene (trimethyl-Phloroglucinol) in HOAc and bubbling through HCl gas, which must cause dehydration of the initially formed 3° alcohol to o,o,p-trimethoxy-MPTP. For the next step, hydroboration of the product from the last step is done by the addition of diborane to the olefin, followed by oxidation of the hydroborane adduct with a peroxide then affords the hydration product as the trans isomer (2). {Although borane is the usual reagent, it appears here that it is generated in situ from BF3 and NaBH4.} The stereochemistry of that hydroxy group is reversed in 2 steps starting by what appears to be a Swern oxidation to a ketone; which is then reduced with NaBH4 to afford (3). The cis relationship is apparently based on the principle that the hydride ion approaches from the less sterically occluded/precluded side, which in this case is opposite to the Ar. 3 is then acylated with acetic anhydride in the presence of BF3. Using an excess of the latter reagent leads to selective desmethylation of the ether adjacent to the newly introduced acetyl function (4). Claisen condensation of that product with methyl-2-chlorobenzoate would then afford the β-diketone (5). A stream of dry HCl gas in a condensation reaction splits out one equivalent of water and formation of the pyran ring by an addition-elimination reaction (6). Desmethylation of the two remaining methoxy groups (phenolic ethers), for example boron tribromide, would then afford alvocidib (7).
...
Wikipedia
