Borane
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Systematic IUPAC name
borane (substitutive)
trihydridoboron (additive) |
|||
Other names
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| 44 | |||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| BH3 | |||
| Molar mass | 13.83 g·mol−1 | ||
| Appearance | colourless gas | ||
| hydrolyses | |||
| Solubility in Ammonia | 3.2 mol L−1 | ||
| Thermochemistry | |||
|
Std molar
entropy (S |
187.88 kJ mol−1 K−1 | ||
|
Std enthalpy of
formation (ΔfH |
106.69 kJ mol−1 | ||
| Structure | |||
| D3h | |||
| trigonal planar | |||
| trigonal planar | |||
| 0 D | |||
| Related compounds | |||
|
Related compounds
|
|||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
| Infobox references | |||
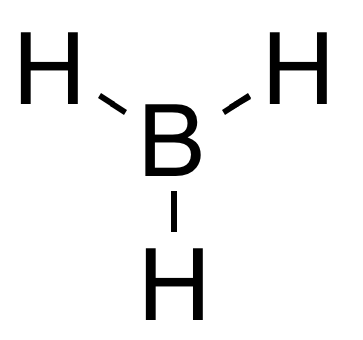
Borane (also systematically named trihydridoboron), also called borine, is an inorganic compound with the chemical formula BH
3. It is a colourless gas that cannot be concentrated in pure form. Borane is both the simplest member of the boranes, and the prototype of the monoboranes.
In 1937, the discovery of carbonyltrihydridoboron, the adduct of borane with carbon monoxide, among other borane adducts, played an important role in exploring the chemistry of "normal" boranes at a time when three-centre two-electron bonding was not yet known. This discovery also implied the existence of borane, however, it was not until some years later that direct evidence was observed.
The matrix isolated products of laser ablated boron atoms with hydrogen forms BH3 as a minor constituent along with B2H6, diborane and BH(H2) complex. Studies of gas phase diborane have detected monomeric BH3.
The structure of BH3 is trigonal planar (D3hmolecular symmetry) with an experimentally determined B–H bond length of 119 pm. This the same as the terminal B–H bond length in diborane(6). The dominant behaviour of borane is its dimerisation to form diborane, as shown by the enthalpy of the reaction, which is predicted to be near -40 kcal/mol. For such an exothermic process, the concentration of BH3 is negligible in solution.
Although borane in principle acts as a Lewis acid, the many 1:1 adducts are invariably prepared from diborane or via ligand exchange of an existing adduct.
The stability sequence of these estimated spectroscopically and thermochemically is:-
BH3 has some soft acid characteristics (sulfur donors are more stable than oxygen donors).
...
Wikipedia


