Zopiclone
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Imovane, Zimovane |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration |
Oral tablets, 3.75 mg (UK), 5 or 7.5 mg |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 75-80% |
| Protein binding | 52–59% |
| Metabolism | Hepatic through CYP3A4 and CYP2E1 |
| Biological half-life | ~5 hours (3.5–6.5 hours) ~7–9 hours for over 65 |
| Excretion | Urine (80%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.051.018 |
| Chemical and physical data | |
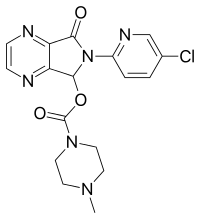
| Formula | C17H17ClN6O3 |
| Molar mass | 388.808 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Zopiclone (brand names Zimovane and Imovane) is a nonbenzodiazepine hypnotic agent used in the treatment of insomnia. It is a cyclopyrrolone, which increases the normal transmission of the neurotransmitter gamma-Aminobutyric acid in the central nervous system, as benzodiazepines do, but in a different way.
As zopiclone is sedating, it is marketed as a sleeping pill. It works by causing a depression or tranquilization of the central nervous system. After prolonged use, the body can become accustomed to the effects of zopiclone. When the dose is then reduced or the drug is abruptly stopped, withdrawal symptoms may result. These can include a range of symptoms similar to those of benzodiazepine withdrawal. Although withdrawal from therapeutic doses of zopiclone and its isomers (i.e. eszopiclone) do not typically present with convulsions and are therefore not considered life-threatening, patients may experience such significant agitation and/or anxiety that they seek emergency medical attention.
In the United States, zopiclone is not commercially available, although its active stereoisomer, eszopiclone, is sold under the name Lunesta. Zopiclone is a controlled substance in the United States, Japan, Brazil, and some European countries, and may be illegal to possess without a prescription. However, it is readily available in other countries where it is marketed under the brand name Imovane, and is not a controlled substance in its available 7.5 mg, 5 mg, and 3.75 mg oral tablet formulations.
Zopiclone is known colloquially as a "Z-drug". Other Z-drugs include zaleplon (Sonata) and zolpidem (Ambien and AmbienCR) and were initially thought to be less addictive and/or habit-forming than benzodiazepines. However, this appraisal has shifted somewhat in the last few years as cases of addiction and habituation have been presented. Zopiclone is recommended to be taken on a short-term basis, usually a week or less. Daily or continuous use of the drug is not usually advised.
...
Wikipedia
