Zinc cyanide
 |
|
| Identifiers | |
|---|---|
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.331 |
|
PubChem CID
|
|
| RTECS number | ZH1575000 |
|
|
|
|
| Properties | |
| Zn(CN)2 | |
| Molar mass | 117.444 g/mol |
| Appearance | white powder |
| Density | 1.852 g/cm3, solid |
| Melting point | 800 °C (1,470 °F; 1,070 K) (decomposes) |
| 0.00005 g/100 mL (20 °C) | |
| Solubility | attacked by alkalies, KCN, ammonia |
| −46.0·10−6 cm3/mol | |
| Hazards | |
|
EU classification (DSD) (outdated)
|
not listed |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
100 mg/kg, rat (intraperitoneal) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Zinc cyanide is the inorganic compound with the formula Zn(CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds.
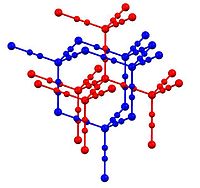
In Zn(CN)2, zinc adopts the tetrahedral coordination environment, all linked by bridging cyanide ligands. The structure consists of two "interpenetrating" structures (blue and red in the picture above). Such motifs are sometimes called "expanded diamondoid" structures. Some forms of SiO2 adopt a similar structure, wherein the tetrahedral Si centres are linked by oxides. The cyanide group shows head to tail disorder with any zinc atom having between one and four carbon neighbours, and the remaining being nitrogen atoms. It shows one of the largest negative coefficients of thermal expansion (exceeding the previous record holder, zirconium tungstate).
Typical for an inorganic polymer, Zn(CN)2 is insoluble in most solvents. The solid dissolves in, or more precisely, is degraded by, aqueous solutions of basic ligands such as hydroxide, ammonia, and additional cyanide to give anionic complexes.
Zn(CN)2 is fairly easy to make by combining aqueous solutions of cyanide and zinc ions, for example via the double replacement reaction between KCN and ZnSO4:
For commercial applications, some effort is made to avoid halide impurities by using acetate salts of zinc:
Zinc cyanide is also produced as a byproduct of certain gold extraction methods. Procedures to isolate gold from aqueous gold cyanide sometimes call for the addition of zinc:
The main application of Zn(CN)2 is for electroplating of zinc from aqueous solutions containing additional cyanide.
Zn(CN)2 is used to introduce the formyl group in to aromatic compounds in the Gatterman reaction where it serves a convenient, safer, and non-gaseous alternative to HCN. Because the reaction uses HCl, Zn(CN)2 also supplies the reaction with ZnCl2 in-situ, a Lewis acid catalyst. Examples of Zn(CN)2 being used in this way include the synthesis of 2-Hydroxy-1-nafthaldehyde and Mesitaldehyde.
...
Wikipedia

