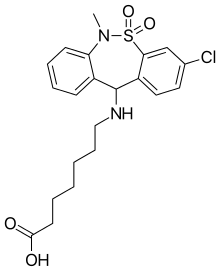
Tianeptine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Coaxil, Stablon |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 99% |
| Protein binding | 95% |
| Metabolism | Hepatic |
| Biological half-life | 2.5-3 hours, 4-9 hours (elderly) |
| Excretion | Renal (65%),Faecal (15%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.131.750 |
| Chemical and physical data | |
| Formula | C21H25ClN2O4S |
| Molar mass | 436.953 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Tianeptine (brand names Stablon, Coaxil, Tatinol, Tianeurax and Salymbra) is a drug used primarily in the treatment of major depressive disorder, although it may also be used to treat asthma or irritable bowel syndrome. Chemically it is a tricyclic antidepressant (TCA), but it has different pharmacological properties than typical TCAs as recent research suggests that tianeptine produces its antidepressant effects through indirect alteration of glutamate receptor activity (i.e., AMPA receptors and NMDA receptors) and release of BDNF, in turn affecting neural plasticity.
Tianeptine has antidepressant and anxiolytic (anti-anxiety) properties with a relative lack of sedative, anticholinergic and cardiovascular adverse effects, thus suggesting it is particularly suitable for use in the elderly and in those following alcohol withdrawal; such persons can be more sensitive to the adverse effects of psychotropic drugs. Recent results indicate possible anticonvulsant (anti-seizure) and analgesic (painkilling) activity of tianeptine via immediate or downstream modulation of adenosine A1 receptors (as the effects could be experimentally blocked by antagonists of this receptor).
...
Wikipedia
