Tartaric acid
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
2,3-Dihydroxybutanedioic acid
|
|
| Other names
Tartaric acid
2,3-Dihydroxysuccinic acid Threaric acid Racemic acid Uvic acid Paratartaric acid |
|
| Identifiers | |
|
526-83-0 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:15674 |
| ChEMBL |
ChEMBL333714 ChEMBL1200861 |
| ChemSpider |
852 |
| DrugBank |
DB01694 |
| ECHA InfoCard | 100.121.903 |
| E number | E334 (antioxidants, ...) |
| KEGG |
C00898 |
| MeSH | tartaric+acid |
| PubChem | 875 |
|
|
|
|
| Properties | |
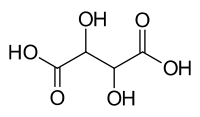
| C4H6O6(Basic formula) HO2CCH(OH)CH(OH)CO2H (Structural formula) |
|
| Molar mass | 150.087 g/mol |
| Appearance | white powder |
| Density | 1.79 g/mL (H2O) |
| Melting point | 171 to 174 °C (340 to 345 °F; 444 to 447 K) (L or D-tartaric; pure) 206 °C (DL, racemic) 165–166 °C ("meso-anhydrous") 146–148 °C (meso-hydrous) |
| 1.33 kg/L (L or D-tartaric) 0.21 kg/L (DL, racemic) |
|
| Acidity (pKa) | L(+) 25 °C : pKa1= 2.89 pKa2= 4.40 meso 25 °C: pKa1= 3.22 pKa2= 4.85 |
| -67.5·10−6 cm3/mol | |
| Hazards | |
|
EU classification (DSD)
|
Irritant(Xi) |
| R-phrases | R36 |
| Related compounds | |
|
Other cations
|
Monosodium tartrate Disodium tartrate Monopotassium tartrate Dipotassium tartrate |
|
Related carboxylic acids
|
Butyric acid Succinic acid Dimercaptosuccinic acid Malic acid Maleic acid Fumaric acid |
|
Related compounds
|
2,3-Butanediol Cichoric acid |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
0.21 kg/L (DL, racemic)
1.25 kg/L ("meso")
Tartaric acid is a white crystalline organic acid that occurs naturally in many plants, most notably in grapes. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of winemaking. It is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant and to impart its distinctive sour taste.
Tartaric is an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics, and is a dihydroxyl derivative of succinic acid.
Tartaric acid was first isolated from potassium bitartrate circa 800 AD, by the alchemist Jābir ibn Hayyān. The modern process was developed in 1769 by the Swedish chemist Carl Wilhelm Scheele.
Tartaric acid played an important role in the discovery of chemical chirality. This property of tartaric acid was first observed in 1832 by Jean Baptiste Biot, who observed its ability to rotate polarized light. Louis Pasteur continued this research in 1847 by investigating the shapes of potassium sodium tartrate crystals, which he found to be chiral. By manually sorting the differently shaped crystals, Pasteur was the first to produce a pure sample of levotartaric acid.
...
Wikipedia
