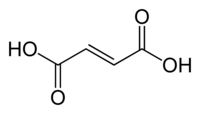
Fumaric acid
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
(2E)-But-2-enedioic acid
|
|
| Other names
Fumaric acid
trans-1,2-Ethylenedicarboxylic acid 2-Butenedioic acid trans-butenedioic acid Allomaleic acid Boletic acid Donitic acid Lichenic acid |
|
| Identifiers | |
|
110-17-8 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:18012 |
| ChEMBL |
ChEMBL503160 |
| ChemSpider |
10197150 |
| DrugBank |
DB04299 |
| ECHA InfoCard | 100.003.404 |
| EC Number | 203-743-0 |
| E number | E297 (preservatives) |
| KEGG |
C00122 |
| UNII |
88XHZ13131 |
|
|
|
|
| Properties | |
| C4H4O4 | |
| Molar mass | 116.07 g·mol−1 |
| Appearance | White solid |
| Density | 1.635 g/cm3 |
| Melting point | 287 °C (549 °F; 560 K) (decomp) |
| 4.9 g/L at 20°C | |
| Acidity (pKa) | pka1 = 3.03, pka2 = 4.44 |
| -49.11·10−6 cm3/mol | |
| Pharmacology | |
| D05AX01 (WHO) | |
| Hazards | |
|
EU classification (DSD)
|
Irritant (Xi) |
| R-phrases | R36 |
| S-phrases | (S2)-S26 |
| NFPA 704 | |
| Related compounds | |
|
Related carboxylic acids
|
maleic acid succinic acid crotonic acid |
|
Related compounds
|
fumaryl chloride fumaronitrile dimethyl fumarate iron(II) fumarate |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Fumaric acid or trans-butenedioic acid is the chemical compound with the formula HO2CCH=CHCO2H. This white crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are trans (E) and in maleic acid they are cis (Z). Fumaric acid has a fruit-like taste.
The salts and esters are known as fumarates. Fumarate can also refer to the C4H2O4−− ion (in solution).
Fumaric acid was first prepared from succinic acid. A traditional synthesis involves oxidation of furfural (from the processing of maize) using chlorate in the presence of a vanadium-based catalyst. Currently, industrial synthesis of fumaric acid is mostly based on catalytic isomerisation of maleic acid in aqueous solutions at low pH. Maleic acid is accessible in large volumes as a hydrolysis product of maleic anhydride, produced by catalytic oxidation of benzene or butane.
The chemical properties of fumaric acid can be anticipated from its component functional groups. This weak acid forms a diester, it undergoes additions across the double bond, and it is an excellent dienophile.
Fumaric acid does not combust in a bomb calorimeter under conditions where maleic acid deflagrates smoothly. For teaching experiments designed to measure the difference in energy between the cis- and trans- isomers, a measured quantity of carbon can be ground with the subject compound and the enthalpy of combustion computed by difference.
...
Wikipedia

