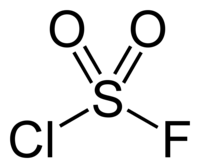
Sulfuryl chloride fluoride
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Sulfuryl chloride fluoride
|
|||
| Other names
Sulfuryl fluoride chloride
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.033.737 | ||
|
PubChem CID
|
|||
| RTECS number | WT4900000 | ||
|
|||
|
|||
| Properties | |||
| ClFO2S | |||
| Molar mass | 118.52 g/mol | ||
| Appearance | colourless gas | ||
| Density | 1.623 g/cm3 at 0 °C | ||
| Melting point | −124.7 °C (−192.5 °F; 148.5 K) | ||
| Boiling point | 7.1 °C (44.8 °F; 280.2 K) | ||
| hydrolyses | |||
| Solubility in other solvents | SO2 and ether | ||
| Structure | |||
| tetrahedral | |||
| Hazards | |||
| Main hazards | moderately toxic, corrosive | ||
| Safety data sheet | "External MSDS" | ||
| R-phrases (outdated) | R14 R34 | ||
| S-phrases (outdated) | S24/25 S26 S27 S28 S36/37/39 S38 S45 | ||
| Related compounds | |||
|
Related compounds
|
SO2Cl2, SO2F2 ClSO2(NCO) |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Sulfuryl chloride fluoride is the chemical compound with the formula SO2ClF. It is employed as a solvent for highly oxidizing compounds.
The laboratory-scale synthesis begins with the preparation of potassium fluorosulfite:
This salt is then chlorinated to give sulfuryl chloride fluoride
Further heating (180 °C) of potassium fluorosulfite with the sulfuryl chloride fluoride gives sulfuryl fluoride.
Alternatively, sulfuryl chloride fluoride can be prepared without using gases as starting materials by treating sulfuryl chloride with ammonium fluoride or potassium fluoride in trifluoroacetic acid.
...
Wikipedia


