Chlorosulfonyl isocyanate
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Chlorosulfonyl isocyanate
|
|
| Other names
N-Carbonylsulfamyl chloride
Chloropyrosulfonyl isocyanate Sulfuryl chloride isocyanate |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.378 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| CNClO3S | |
| Molar mass | 141.53 g/mol |
| Appearance | colorless liquid |
| Density | 1.626 g/cm3 |
| Melting point | −44 °C (−47 °F; 229 K) |
| Boiling point | 107 °C (225 °F; 380 K) |
| decomposition | |
| Solubility in other solvents | Chlorocarbons MeCN |
|
Refractive index (nD)
|
1.447 |
| Structure | |
| tetrahedral at S | |
| Hazards | |
| Main hazards | toxic, corrosive, flammable, reacts violently with water |
| Safety data sheet | "External MSDS" |
| R-phrases (outdated) | R14 R20 R24/25 R29 R34 R42/43 |
| S-phrases (outdated) | (S1/2) S8 S24 S26 S30 S36/37/39 S38 S45 |
| NFPA 704 | |
| Related compounds | |
|
Related compounds
|
Thionyl chloride Cyanogen bromide Phosphoryl chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Chlorosulfonyl isocyanate is the chemical compound ClSO2NCO, known as CSI. This compound is a versatile reagent in organic synthesis.
CSI is prepared by treating cyanogen chloride with sulfur trioxide, the product being distilled directly from the reaction mixture.
In this transformation, both the carbon and the nitrogen termini of CN are functionalized.
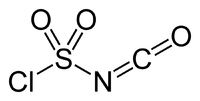
The structure of CSI is represented as ClS(O)2-N=C=O. It consists of two electron-withdrawing components, the chlorosulfonyl group (SO2Cl) and the isocyanate group (-N=C=O). Because of its resulting electrophilicity, the use of CSI in chemical synthesis requires relatively inert solvents such as chlorocarbons, acetonitrile, and ethers.
The molecule has two electrophilic sites, the carbon and the S(VI) center.
CSI has been employed for the preparation of β-lactams, some of which are medicinally important. Thus, alkenes undergo a [2+2]-cycloaddition to give the sulfonamide. The SO2Cl group can be removed simply by hydrolysis, leaving the secondary amide. Other reactions of CSI:
CSI is toxic, corrosive and reacts violently with water. It cannot be stored in glass-stoppered flasks, requiring instead polyethylene bottles.
...
Wikipedia

