Substituted phenethylamines
| Substituted phenethylamine | |
|---|---|
| Drug class | |
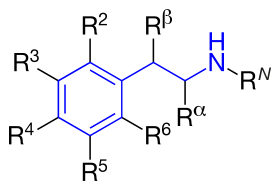
The structural formula of phenethylamine with marked substitution points. Phenethylamine is obtained when
R2=R3=R4=R5=R6=RN=Rα=Rβ=H. |
|
| Class identifiers | |
| Chemical class | Substituted derivatives of phenethylamine |
Substituted phenethylamines (or simply phenethylamines) are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents.
The structural formula of any substituted phenethylamine contains a phenyl ring that is joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. Hence, any substituted phenethylamine can be classified according to the substitution of hydrogen (H) atoms on phenethylamine's phenyl ring, sidechain, or amino group with a specific group of atoms.
Many substituted phenethylamines are psychoactive drugs which belong to a variety of different drug classes, including central nervous system stimulants (e.g., amphetamine), hallucinogens (e.g., dl-2,5-dimethoxy-4-methylamphetamine a.k.a. DOM), entactogens (e.g., 3,4-methylenedioxyamphetamine a.k.a. MDA), appetite suppressants (e.g. phentermine), nasal decongestants and bronchodilators (e.g., levomethamphetamine and pseudoephedrine), antidepressants (e.g. bupropion and phenelzine), antiparkinson agents (e.g., selegiline), and vasopressors (e.g., ephedrine), among others. Many of these psychoactive compounds exert their pharmacological effects primarily by modulating monoamine neurotransmitter systems; however, there is no mechanism of action or biological target that is common to all members of this subclass.
...
Wikipedia
