Selegiline
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | se-LE-ji-leen |
| Trade names | Pill form is generic and available under many brand names;transdermal patch is called Emsam |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697046 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral, transdermal, buccal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 4.4% (oral, fasted), 20% (oral, after food), 73% (patch) |
| Protein binding | ≥85–90% |
| Metabolism | Extensive in liver |
| Metabolites | N-desmethylselegiline, L-amphetamine and L-methamphetamine |
| Biological half-life | 10 hours (oral), 18–25 hours (transdermal) |
| Excretion | Urine (main route) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.109.269 |
| Chemical and physical data | |
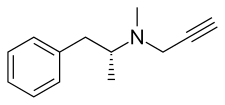
| Formula | C13H17N |
| Molar mass | 187.281 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Selegiline, also known as L-deprenyl, is a substituted phenethylamine. At normal clinical doses, it is a selective irreversible MAO-B inhibitor. In larger doses it loses its specificity and also inhibits MAO-A. It is available in pill form under many brand names and is used to reduce symptoms in early-stage Parkinson's disease. A transdermal patch (brand name, Emsam) is used to treat depression.
For all human uses and all forms, selegiline is Pregnancy Category C, meaning that caution is in order because studies in pregnant laboratory animals have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but that the drug's potential benefits may nonetheless warrant use of the drug in some pregnant women.
In its pill form, selegiline is used to treat symptoms of Parkinson's disease. It can be used on its own or in a combination with another agent, most often L-DOPA.
Selegiline delays the time point when the L-DOPA (levodopa) treatment becomes necessary from about 11 months to about 18 months after diagnosis, which is beneficial despite not being definitive evidence of neuroprotection. The rationale for adding selegiline to levodopa is to decrease the required dose of levodopa and thus reduce the motor complications of levodopa therapy.
Selegiline is also delivered via a transdermal patch; in this form it is used as a treatment for major depressive disorder.
A quantitative review published in 2015 found that for the pooled results of the pivotal trials, the number needed to treat (a sign of effect size, so a low number is better) for the patch for symptom reduction was 11, and for remission, was 9. The number needed to harm (inverse of the NNT, a high number here is better) ranged from 387 for sexual side effects to 7 for application site reaction. With regard to the likelihood to be helped or harmed (LHH), the analysis showed that the selegiline patch was 3.6 times as likely to lead to a remission vs. a discontinuation due to side effects; the LHH for remission vs. incidence of insomnia was 2.1; the LHH for remission vs. discontinuation due to insomnia was 32.7. The LHH for remission vs insomnia and sexual dysfunction were both very low.
...
Wikipedia
